Venkatesh Aiyagari, Sean Ruland, Philip B. Gorelick
Neurogenic Hypertension, Including Hypertension Associated with Stroke or Spinal Cord Injury
The nervous system and blood pressure (BP) are closely interrelated.1 It is well recognized that the elevated BP response to stressors is mediated by the sympathetic nervous system (SNS). However, the role of the SNS in the long-term regulation of BP and the initiation and maintenance of hypertension is also increasingly appreciated. Several studies of plasma catecholamine levels, renal norepinephrine spillover, microneurography, and heart rate variability support the hypothesis that sympathetic activation plays a major role in hypertensive patients.2 The SNS also has an important role in hypertension after neurologic injury. This chapter describes the physiology and management of hypertension in such injury.
Physiology and Pathophysiology
Neural Control of Blood Pressure
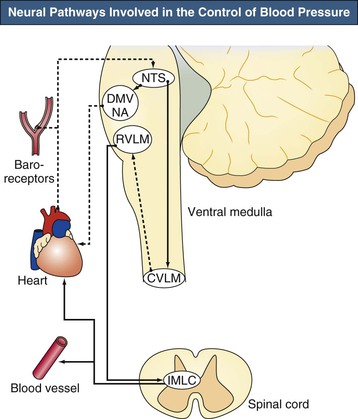
The brainstem, especially the ventral medulla, has a key role in the maintenance of BP (Fig. 42-1). BP is controlled by the nucleus tractus solitarius (NTS), which receives inhibitory baroreceptor afferents, and by the rostral ventrolateral medulla and rostral ventromedial medulla, which are the source of excitatory descending bulbospinal pressor pathways. In addition, a depressor center in the caudal ventrolateral medulla composed of γ-aminobutyric acid (GABA)–containing neurons receives afferents from the NTS and projects to the rostral ventral medulla. These inhibitory GABA-containing neurons are tonically active, and reduced activity of these neurons leads to hypertension.3–5
The ultimate effector units are the sympathetic neurons located in the intermediolateral cell column of the spinal cord and the parasympathetic neurons found in the dorsal motor nucleus of the vagus and nucleus ambiguus located in the medulla. In addition, impulses from the limbic system, cerebral cortex, and hypothalamus also directly or indirectly project to the intermediolateral cell column of the spinal cord and influence BP regulation.
The factors that lead to increased sympathetic activation in hypertension are poorly understood. In recent years, however, the role of low-grade inflammation and reactive oxygen species (ROS) has been recognized. Some studies also suggest that activation of low-grade inflammation in the kidney may activate afferent sympathetic nerves that stimulate the central SNS. Hypertension is also associated with increased levels of circulating inflammatory markers such as tumor necrosis factor α, interleukin-6, C-reactive protein, monocyte chemoattractant protein 1, and adhesion molecules such as P-selectin and intercellular adhesion molecule 1. Angiotensin II (Ang II) and aldosterone also play a crucial role in vascular inflammation, and both candesartan and mineralocorticoid antagonists have been shown to decrease the levels of inflammatory markers. In addition, Ang II–mediated hypertension has been associated with brain microglial activation and increased brain levels of inflammatory cytokines and ROS. An increase in ROS may directly activate or sensitize sympathetic neurons and scavenge nitric oxide, which tonically inhibits sympathetic outflow. Thus, a dysfunction of the neural-immune-vascular triad leading to an increase in central oxidative stress may be the driving force behind sympathetic activation, which then increases Ang II and promotes further inflammation and vascular dysfunction.6
Cerebrovascular Autoregulation
Under normal conditions, cerebral blood flow (CBF) of the adult brain is 50 ml/100 g/min. CBF is regulated by the following relationship between cerebral perfusion pressure (CPP) and cerebrovascular resistance (CVR):
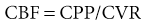
Cerebral perfusion pressure is defined as the difference between the mean arterial pressure (MAP) and the intracranial pressure (ICP). If ICP is increased, systemic BP needs to be higher to maintain CPP and CBF.
Cerebrovascular autoregulation maintains a constant blood flow over a wide range of CPP. Normally, changes in BP have little effect on CBF because of compensatory changes in cerebrovascular resistance. An increase in BP produces vasoconstriction, and a decrease in BP produces vasodilation, thus keeping CBF constant (Fig. 42-2). Autoregulation is effective over a range of CPP from about 60 to 150 mm Hg. In chronically hypertensive individuals, the cerebral arterioles develop medial hypertrophy and lose the ability to dilate effectively at lower pressures. This causes the autoregulatory curve to shift to the right.7 In these individuals, a rapid reduction of BP may lead to a drop in CBF even though the BP might still be within the “normal” range. With effective control of hypertension for several months, the normal range for autoregulation can be reestablished.8
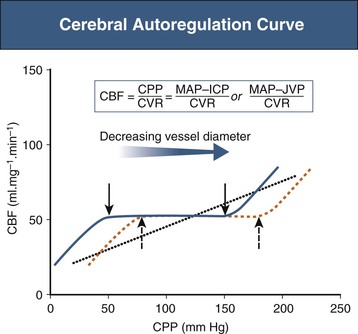
Above the upper limit of autoregulation, there is breakthrough vasodilation leading to damage of the blood-brain barrier and cerebral edema and possibly cerebral hemorrhage. Below the lower limit of autoregulation, decreases in CPP lead to a decrease in CBF. Under these circumstances, increased extraction of oxygen and glucose maintains normal cerebral metabolism and brain function. When CBF decreases to less than 20 ml/100 g/min, the increase in oxygen extraction is no longer able to supply the metabolic needs of the brain, leading to impaired brain function.
Hypertension After Stroke
Epidemiology
Hypertension is the most important modifiable risk factor for cerebrovascular accident (stroke), and reduction in BP is effective in the primary prevention of stroke, improves outcomes in patients who have had an ischemic stroke, and may be especially beneficial for lowering risk of stroke among those with a history of intracerebral brain hemorrhage.9 Combined data from 40 trials of antihypertensive agents have demonstrated that a 10% reduction in systolic BP lowers stroke risk by one third.10 A 5-mm lower diastolic together with a 9-mm lower systolic BP confers a 33% lower risk of stroke, and a 10-mm lower diastolic together with an 18- to 19-mm lower systolic confers more than a 50% reduction in stroke risk.11 In patients who had a stroke, the Perindopril Protection against Recurrent Stroke Study (PROGRESS) showed that a reduction in BP was associated with a significant reduction of total stroke recurrence by 28% and a reduction of major coronary and vascular events by 26%, even in patients with a normal initial BP.12
However, the management of BP in the immediate aftermath of a stroke is controversial.13 A high proportion of patients have elevated BP immediately after a stroke, and BP has been shown to decrease spontaneously over 1 to 2 weeks to the prestroke baseline in most patients. Box 42-1 lists some postulated causes of elevated BP. Increased BP after stroke is associated with higher mortality. Nonetheless, it is uncertain whether the increased BP directly contributes to poor outcome, or whether immediate lowering of BP will lead to better outcomes.
Pathophysiology
An understanding of cerebrovascular pathophysiology is essential to understand the pros and cons of treating hypertension in these patients (Table 42-1).
Table 42-1
Advantages and disadvantages (pros and cons) of acute treatment of hypertension in stroke.
BP, Blood pressure; CPP, cerebral perfusion pressure; ICP, intracranial pressure; tPA, tissue plasminogen activator.
| Acute Treatment of Hypertension in Stroke: Advantages and Disadvantages | |
| Advantages | Disadvantages |
| Acute Ischemic Stroke | |
Might decrease stroke progression Might decrease hemorrhagic transformation (especially after tPA) Might decrease cerebral edema formation Might be helpful for systemic reasons (e.g., associated myocardial ischemia) Patients likely to be more compliant with antihypertensive use if treatment initiated in hospital | |
| Acute Intracerebral Hemorrhage | |
| Aneurysmal Subarachnoid Hemorrhage | |
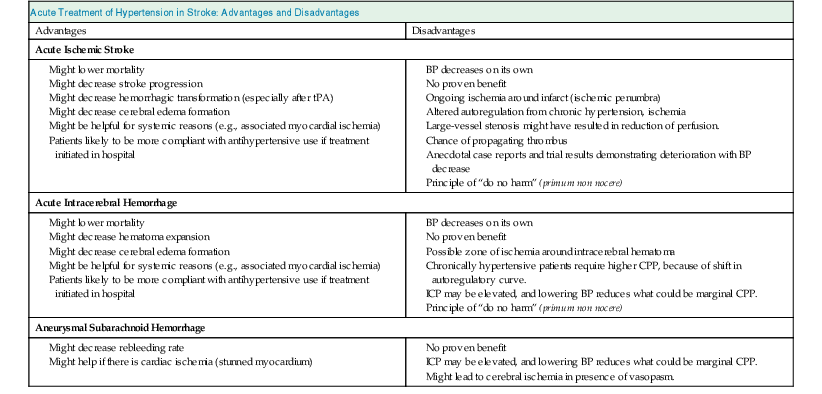
In patients with an ischemic stroke, vascular occlusion leads to a central region of irreversibly ischemic brain surrounded by an ischemic zone where blood flow is reduced but brain tissue is still viable. After 2 or 3 days, the ischemic areas either recover completely or undergo infarction. In the first few days, perfusion in this zone is marginal, and a further decrease in blood flow might lead to infarction. Because cerebral autoregulation is impaired with acute ischemic stroke, a fall in BP could lower blood flow and extend infarction, and a very high BP could lead to hemorrhagic transformation, especially if thrombolytic agents have been given.
At times, it can be difficult to distinguish between hypertensive encephalopathy, in which lowering of BP is clearly indicated, and ischemic stroke with hypertension. The level of consciousness, the presence of focal neurologic deficits, and the ophthalmoscopic (funduscopic) examination can help make this distinction. Hypertensive encephalopathy is a syndrome of global neurologic dysfunction, usually with papilledema, and focal neurologic deficits are usually less prominent. In acute ischemic stroke, the focal neurologic deficit is more prominent, and alterations of consciousness are less common, except with brainstem strokes or “malignant” brain edema from massive hemispheric infarction.
In patients with intracerebral hemorrhage, the considerations are different.14 Hematoma expansion occurs in one third of patients, with an intracerebral hemorrhage in the first 24 hours.15 Therefore, BP is often lowered in these patients to decrease hematoma expansion. However, the evidence does not support a clear association between high BP and hematoma expansion.16 On the other hand, some patients with intracerebral hemorrhage might have increased ICP from the hematoma volume or associated hydrocephalus. In these patients, lowering of BP is not warranted because it might critically lower CPP; monitoring of ICP and CPP may be helpful.
Patients with aneurysmal subarachnoid hemorrhage have significant risk of rebleeding; tight BP control is recommended to decrease the risk. Some patients with subarachnoid hemorrhage have associated myocardial dysfunction (“stunned myocardium”), in which case high BP might worsen myocardial function. Again, in patients with hydrocephalus or an associated intracerebral hemorrhage, monitoring ICP and CPP can help guide BP management. In the latter half of the first week and in the second week after subarachnoid hemorrhage, many patients develop vasospasm of the intracranial arteries. Reduction of BP may lead to worsening of cerebral ischemia in this situation. Therefore, once the aneurysmal rupture has been adequately treated with surgical clipping or coiling, BP is usually maintained at a normal or slightly elevated level in these patients.
Diagnosis and Treatment
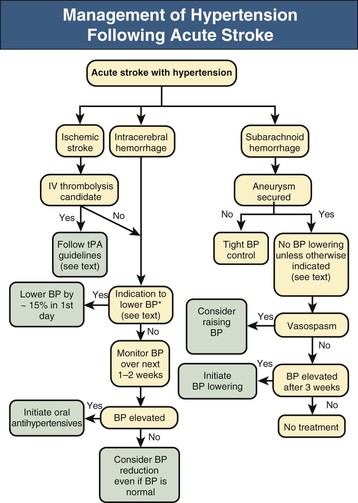
Definitive studies adequately powered to assess the benefits and risks of BP lowering after ischemic and hemorrhagic stroke have not yet been performed. A Cochrane review concluded that evidence is insufficient to evaluate the effect of altering BP on outcome during the acute phase of stroke.17 The following sections summarize the available evidence. Figure 42-3 outlines recommendations for treating BP in different clinical situations.
Acute Ischemic Stroke
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree