Ashley B. Irish
Myeloma and the Kidney
Myeloma is an uncommon hematologic malignancy, accounting for 1% of total and 10% of hematologic malignancies. African Americans have twice the incidence of Caucasians, and males predominate over females. It is a disease of the elderly, with a median age of diagnosis of older than 65 years. The characteristic feature of myeloma is the dysregulated overproduction of immunoglobulin (Ig), and especially the light-chain (LC) component, which can be nephrotoxic. Various causes and manifestations of acute kidney injury (AKI) are possible in myeloma (Table 65-1), but the major risk is myeloma cast nephropathy (MCN), which is a medical emergency that requires prompt diagnosis and intervention to prevent irreversible renal failure.
Etiology and Pathogenesis of Myeloma
Myeloma is a B cell–derived malignancy of incompletely differentiated plasma cells that have two prominent features: increased production of monoclonal Ig, and bone destruction. Normally plasma cells derive from mature uncommitted B cells and after antigen stimulus undergo heavy chain class switching from µ (IgM) expression to α, δ, ε, or γ. Whole Ig production requires the intracellular assembly of two heavy chains and two LCs, kappa (κ) or lambda (λ), to derive whole IgG, IgA, IgD, and IgE immunoglobulin. Normally LCs are excreted in slight excess, with a κ:λ ratio of approximately 2 : 1. In myeloma a clone of cells secretes excessive quantities of a specific Ig and/or LC (the paraprotein or M protein). The genetic and somatic abnormalities underlying this malignant clone are complex and remain incompletely understood but have important implications for prognosis and treatment.1 Both deregulation of cell cycling and impaired apoptosis account for their progressive and dysfunctional accumulation within the bone marrow, and occasionally other organs. Plasma cells express little surface Ig and are recognized by surface expression of CD38 and CD138; they normally reside only in the bone marrow. In myeloma, unrestrained plasma cell growth is supported by a complex milieu of autocrine and paracrine cytokines, especially interleukin (IL)–6. These cytokines are secreted from stromal cells, endothelial cells, and/or osteoclasts and maintain myeloma cell growth, survival, and migration; they also contribute to local organ dysfunction—for example, bone resorption, fracture, and anemia.2
Etiology and Pathogenesis of Renal Disease
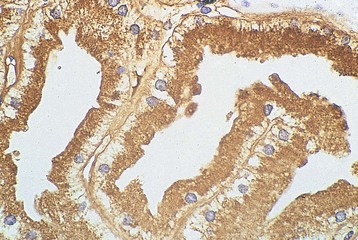
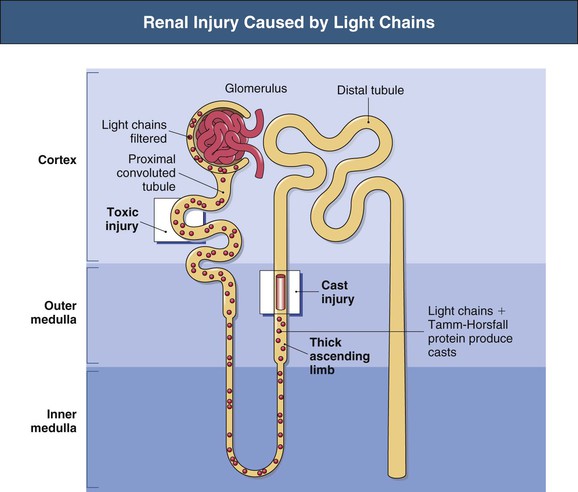
Free light chains (FLCs) circulate as monomers (predominantly κ, approximately 25 kD) and dimers (predominantly λ, approximately 50 kD) with a very short half-life (2 to 6 hours) because of free glomerular filtration, whereas the much larger whole Ig circulates intact for several weeks. The filtered FLC is reabsorbed in the proximal tubule cell (PTC) by receptor-mediated endocytosis after binding with the glycoprotein receptor cubilin (Fig. 65-1).3 LCs in excess can induce an inhibitory effect on endocytosis in vitro and are associated with lysosomal overload and rupture, releasing enzymatic contents into the cytosol, manifesting histologically by evidence of crystallization, vacuolation, and desquamation of the PTC. Endocytosis of light chains induces the release of the proinflammatory cytokines IL-6, IL-8, and monocyte chemoattractant protein 1 (MCP-1) via the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in the PTC.4 This mechanism suggests that LC overload induces factors promoting interstitial injury and fibrosis, as described in other proteinuric states. LC may also be cytotoxic to the PTC via direct DNA injury and the induction of apoptosis.5 Less common manifestations of PTC injury include Fanconi syndrome, which is invariably associated with specific variant κ LCs and often with pathologic evidence of crystalline inclusions.6
Injury to the PTC allows escape or overflow of LCs to the distal nephron where they can interact with the Tamm-Horsfall protein (THP) secreted by the cells of the thick ascending loop of Henle (TAL). Variations in the specificity of the complementarity-determining region 3 (CDR3) of different LCs modify the affinity of the LC for binding with the specific single FLC-binding domain of THP, which could in part explain the variable nephrotoxicity (cast formation) of different LCs.7 This specificity of the individual LC for THP was illustrated by the finding that the intraperitoneal installation of LC isolated from humans with specific renal LC-associated disease induces the same renal injury in animals.8 Although renal injury occurs only in the presence of urinary LC, not all LCs are associated with injury, and neither the amount nor the type of urinary LC correlates with the severity of cast formation. Nevertheless, in general, the higher the urinary excretion of LC, the greater the risk of renal failure and reduced response to chemotherapy.9–11 In addition to LC-specific factors, tubular solute composition and tubular flow rates modulate the risk for cast formation. In animals, urinary acidification, furosemide, increased urinary sodium increased urinary calcium concentration may affect the tendency to increased binding or aggregation of LC with THP, whereas colchicine may reduce this tendency in animals but not in humans.12,13 The formation and passage of casts distally can occlude the tubule and allow intratubular obstruction, with rupture and even backflow of contents (Fig. 65-2).
Epidemiology
Most myeloma presents de novo, although a small number of cases evolve in patients with monoclonal gammopathy of undetermined significance (MGUS) each year. In patients with newly diagnosed myeloma, the prevalence of IgG, IgA, IgD, and FLC-only myeloma was 52%, 21%, 2%, and 16%, respectively.9 IgM and IgE myeloma are extremely uncommon. Approximately 70% of patients with myeloma also have a urinary M protein. At the time of diagnosis of myeloma, up to 50% of patients have evidence of impaired renal function judged by increased serum creatinine; approximately 25% present with serum creatinine exceeding 2 mg/dl (177 µmol/l).9,10 In unselected series, 2% to 10% of patients present with severe renal failure necessitating dialysis; this figure is higher in series reported from renal units. In contrast to the general distribution of M-protein types in myeloma, LC and IgD myeloma are particularly associated with the risk of renal disease, being present in nearly 50% of patients with severe renal disease who require dialysis.14
Clinical Presentation
Most patients present with constitutional symptoms (fatigue, weight loss) and skeletal pain, especially back pain. Renal impairment is common and has a variety of causes (see Table 65-1). In a smaller proportion of patients, renal failure is the presenting manifestation of myeloma, and the diagnosis of myeloma is made or suggested by the renal biopsy. In general these patients have more advanced disease with high morbidity and mortality.14 Renal findings are nonspecific, usually including normal size kidneys and bland urine. Urinary total protein excretion may be markedly increased because of the presence of LCs (Bence Jones protein), whereas urinary dipstick or albumin quantification may be misleading because they measure albumin only. This discrepancy between albumin and total protein measurements may alert clinicians to the diagnosis of myeloma. Normal or elevated ionized calcium, a decreased serum anion gap, lytic bone lesions on x-ray examination, hypogammaglobulinemia or reductions in levels of other immunoglobulin classes (immune paresis), an abnormal serum FLC ratio, and significant cytopenias or blood film changes (plasma cells and/or leukoerythroblastic film) are suggestive of myeloma. Clinical and laboratory findings that may distinguish MCN from other monoclonal immunoglobulin deposition diseases (MIDD) are listed in Table 65-2 and discussed in Chapter 27.
Table 65-2
Clinical features of myeloma kidney versus MIDDs.
LCDD, Light-chain deposition disease.
| Differentiating Features of Myeloma Kidney and Other Monoclonal Immune Deposition Diseases (MIDDs) | ||
| Myeloma Kidney | Other MIDDs | |
| Proteinuria | <3 g/l | >3 g/l |
| Hematuria | Rare | LCDD, occasional Amyloidosis, rare |
| Hypercalcemia | Common | Absent |
| Hypertension | Uncommon | LCDD, common Amyloidosis, uncommon |
| Cytopenias | Anemia very common Leukopenia and thrombocytopenia, occasional | Uncommon |
| Immunoparesis* | Very common | Uncommon |
| Lytic bone lesions | Very common | Absent |
| Renal impairment | Common | Common |
| Associated whole immunoglobulin | IgA, IgD, IgG | None |
| Type of light chain | Either | Amyloid λ > κ LCDD κ > λ |
| Serum light chain elevation | >500 mg/l | <500 mg/l |
* Defined as a reduction in the nonparaprotein globulin fractions.
Pathology
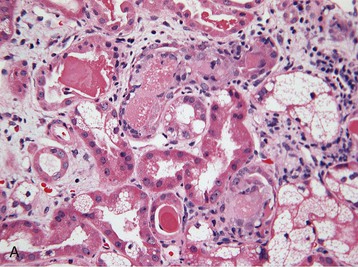
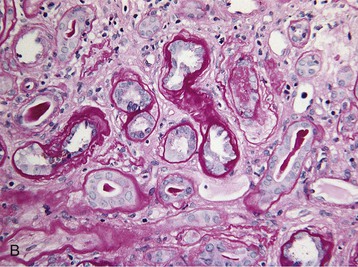
Histologic examination of the kidney in myeloma has diagnostic and prognostic utility, although it is not always required, and the risk of complications after biopsy may be increased in patients with AKI who require dialysis. Biopsy is useful for the initial evaluation of AKI and to guide treatments designed to reverse AKI. Table 65-3 lists the likely findings and prevalence of renal injury noted at histologic or autopsy sampling. MCN is the commonest histologic finding, occurring in 30% to 50% of patients (Box 65-1 and Fig. 65-3, A). MCN is characterized by many distal tubular casts, which are strongly eosinophilic and consist of the monoclonal LC and laminated THP, which often appear fractured after fixation. Casts promote local inflammation with giant cell formation.15,16 In 30% of patients, cast formation may not be prominent despite extensive tubulointerstitial injury (Fig. 65-3, B and C).17 Glomeruli are usually spared unless there is associated LC deposition disease or amyloidosis (see Chapter 27).
Table 65-3
Renal disease in patients with multiple myeloma.
FSGS, Focal segmental glomerulosclerosis.
| Renal Disease in Patients with Multiple Myeloma | |
| Histologic Finding | Prevalence |
| Myeloma kidney (myeloma cast nephropathy) | 30%-50% |
| Interstitial nephritis or fibrosis without cast nephropathy | 20%-30% |
| Amyloidosis | 10% |
| Light-chain deposition disease | 5% |
| Acute tubular necrosis | 10% |
| Other (uric acid nephropathy, tubular crystals, hypercalcemia, FSGS) | 5% |
(From references 26, 66 to 71.)