Fig. 14.1
Port placement for proximal small intestinal resection: right upper quadrant 5-mm port, 5-mm or 12-mm umbilical camera port, a left lower quadrant 5-mm assistant port, and a 12-mm right lower quadrant port
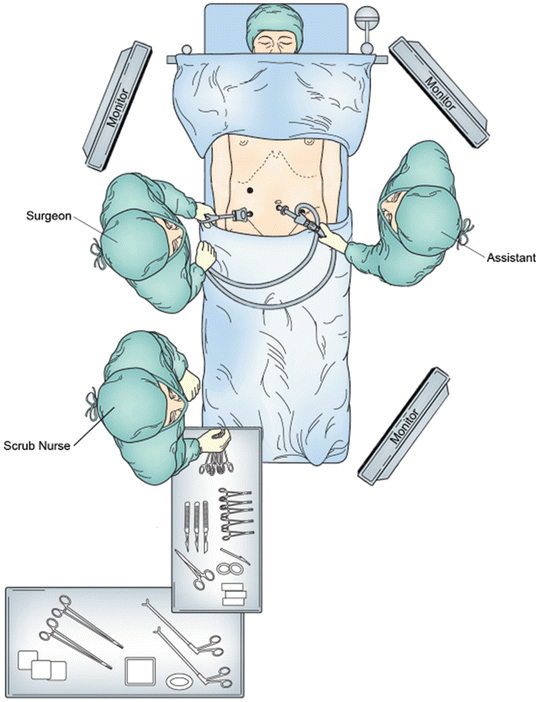
Fig. 14.2
For proximal small bowel pathology, the surgeon stands on the right side of the patient and the assistant stands on the left side. The monitors are placed at the head and foot of the patient
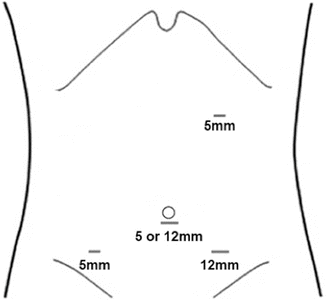
Fig. 14.3
For resection of distal small bowel the ports are placed in a configuration that is the mirror image of Fig. 14.1. There is a left upper quadrant 5-mm port, 5-mm or 12-mm umbilical camera port, a right lower quadrant 5-mm assistant port, and a 12-mm left lower quadrant port
Following port placement, we assess the liver and the rest of the abdominal cavity for any significant pathology or metastatic spread. The patient is placed in Trendelenburg position and the transverse colon elevated. The ligament of Treitz is then located. The patient is positioned back in reverse Trendelenburg position. The small bowel is run from the ligament of Treitz to the ileum carefully looking for synchronous pathology. The location of the tumor is identified and confirmed. Next, we identify the proximal and distal resection margins of the tumor. In cases of suspected malignancy we recommend 5 cm distal and proximal margins of resection with a broad mesenteric resection. The mesentery is marked along the site of proposed division. The small bowel mesentery is then elevated and mesenteric windows are created in an avascular plane beneath the bowel using an energy device. We then place a laparoscopic linear stapler through the mesenteric window and across the proximal bowel (Fig. 14.4). Similar transection is performed for the distal margin. The mesentery is then divided using an energy device (Fig. 14.5). The two intestinal ends are held together and two stay sutures are placed to align the bowel. Enterotomies are created and the two jaws of the laparoscopic linear stapler are negotiated carefully into the limbs of the small intestine (Fig. 14.6). The stapler is fired thus creating a side-to-side/functional end-to-end anastomosis. The enteric defect can be closed with a linear stapler or using hand-sewn technique. An important component of the operation is to close the mesenteric defect to prevent the development of an internal hernia. The specimen is extracted using a specimen retrieval bag through either the 12-mm port or natural orifice (transvaginal) in selected cases [19]. After making sure that there is good hemostasis, the 12-mm port fascial incision is closed with an absorbable suture to prevent a port site hernia.
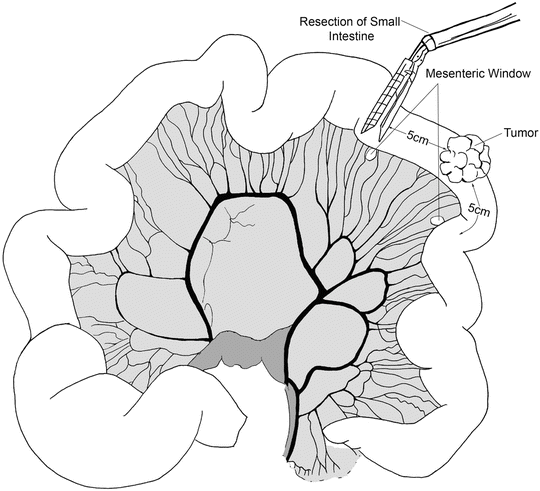
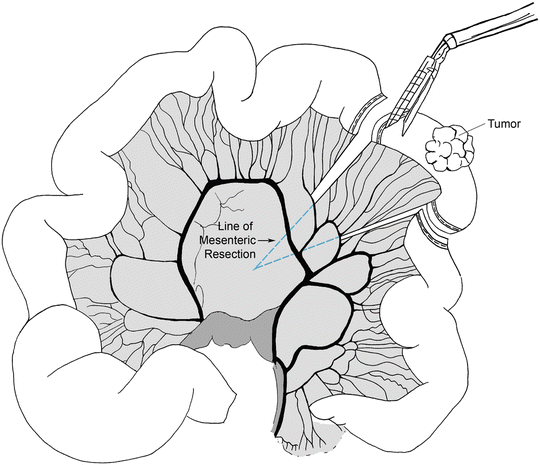
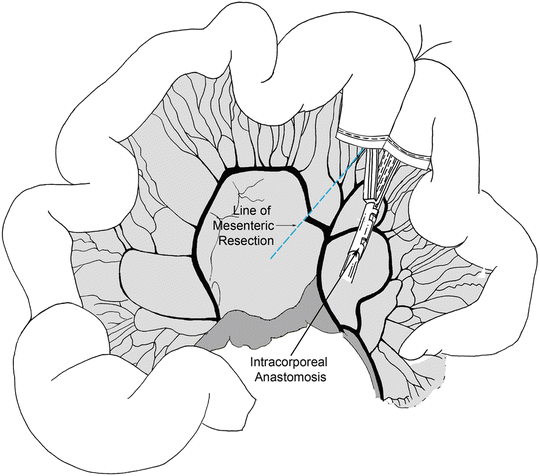

Fig. 14.4
The location of the tumor is identified and mesenteric windows are created in an avascular plane beneath the bowel using an energy device

Fig. 14.5
A laparoscopic linear stapler is placed through the mesenteric window and across the proximal bowel

Fig. 14.6
The two intestinal ends are held together and two stay sutures are placed to align the bowel. Enterotomies are created and the two jaws of the laparoscopic linear stapler are negotiated carefully into the limbs of the small intestine creating a side-to-side/functional end-to-end anastomosis
If extracorporeal anastomosis is planned, the initial steps of access and localization of the pathologic segment of intestine are the same. The abdominal incision (and 12-mm port) may be placed at a periumbilical (proximal small intestine), Pfannenstiel (mid to distal intestine), or right lower quadrant (distal intestine) site. A 4-cm incision is made at one of these sites and a wound protector or laparoscopic hand-port is placed. The affected portion of the intestine is exteriorized through the incision, the mesentery is divided with an energy source, the intestine is divided with a linear stapler, and a stapled anastomosis is performed similar to the laparoscopic technique. The mesenteric defect is sutured closed and the intestine is replaced into the abdominal cavity. The abdominal incision is closed.
Postoperative Management
Postoperatively the patient is extubated and the orogastric or nasogastric tube is usually removed in the operating room. Unless contraindicated, an intravenous dose of ketorolac is given and may be continued every 6 h for 24–48 h. Patients receive a patient controlled anesthesia device for pain control, ondansetron for nausea, and 24 h of parenteral antibiotics. Subcutaneous heparin and SCDs are continued until discharge. The patient is ambulated out of bed beginning the evening after operation. If not removed in the operating room, the Foley catheter is removed on postoperative day one. If not nauseated, clear liquids are instituted on the first postoperative day and the diet is advanced to a regular diet by postoperative day two. Once tolerating a regular diet, pain control is continued with oral narcotic analgesics. The patient is usually ready for discharge by postoperative day three or four.
Complications
Common complications that are seen after laparoscopic small intestinal resection include surgical site infections (superficial and deep), postoperative ileus/ mechanical obstruction, deep venous thrombosis, and bleeding. Anastomotic leak or breakdown occurs in 0.5–1 % of small bowel anastomoses [20–22] and is one of the most dreaded complications of intestinal surgery. Other less common complications include solid organ or viscus injury, anastomotic stricture, and short bowel syndrome when extensive intestinal resection is required.
Prolonged postoperative ileus occurs less frequently following laparoscopic bowel resection compared with open operation [23, 24]. Whenever there is a delay in return of normal bowel function, other complications should be suspected. A mechanical cause for the obstruction, deep space infection, or anastomotic leak all should be excluded before attributing a delay in return of bowel function to postoperative ileus. In cases of true postoperative ileus, management includes NPO (±NGT decompression), hydration, correction of electrolytes, reducing or stopping narcotic medication, sugarless chewing gum, and use of motility agents such as metoclopramide or erythromycin.
Summary
Laparoscopic surgery has gained wide acceptance in the armamentarium of surgeons performing gastrointestinal operations. The potential advantages of the minimally invasive approach to small intestinal neoplasm resection include comparable surgical and oncologic outcomes [3, 25, 26], decreased length of stay [25–28], cosmetically appealing scar, and improved pain scores [24, 28–31]. In addition, when compared with open operations there is a decrease in incisional hernia occurrence, diminished adhesion formation with a presumed lower postoperative bowel obstruction incidence [32]. All of these advantages should translate to improved patient satisfaction with a decrease in overall health care costs [30]. This type of operation will gain more popularity in the coming years as a greater number of surgeons become comfortable with minimally invasive surgical approaches.
Key Operative Steps
1.
Access peritoneal cavity by Veress needle or open (Hasson) approach.
2.
Identify the small bowel segment to be resected.
3.
Elevate the transverse colon, locate the ligament of Treitz, and run the small bowel.
4.
Create a mesenteric window with 5 cm proximal and distal margins.
5.
Divide the proximal intestine with a laparoscopic linear stapler.
6.




Divide the mesentery with an endoscopic bipolar or ultrasonic energy device.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








