Fig. 2.1
Electron microscopy of a glomerulus from a renal biopsy of a patient with minimal change disease, showing diffuse foot process effacement (white arrows) and microvillous degenerative changes (black arrows) of the visceral epithelial cells (×2,900). Courtesy of Sanjeev Sethi, MD
Most children with the nephrotic syndrome are not biopsied; instead, they are typically treated empirically with steroids. However, most adult patients with the nephrotic syndrome are biopsied. Hence this chapter will deal with biopsy-proven, adult-onset MCD.
Pathophysiology
The pathophysiology of minimal change disease is not well understood. The animal model which most closely resembles MCD is puromycin aminonucleoside nephrosis (PAN) in rats. Administration of puromycin aminonucleoside to rats causes the production of reactive oxygen species which leads to direct DNA damage. This alters the podocyte actin cytoskeleton, resulting in foot process effacement, detachment from the glomerular basement membrane, and proteinuria. This effect is dose-dependent, and the podocyte changes and proteinuria spontaneously reverse if doses are limited [2].
Regulatory T-Cell Dysfunction
Shalhoub was the first to propose that “lipoid nephrosis is produced by a systemic abnormality of T-cell function resulting in the secretion of a circulating chemical mediator toxic to an immunologically innocent glomerular basement membrane.” This theory was based on several observations: lack of a humoral antibody response, remission induced by measles and steroids/cyclophosphamide (which modify cell-mediated immunity), and the occurrence in Hodgkin’s lymphoma [3].
Many cases of idiopathic nephrotic syndrome remit spontaneously, and significant transient albuminuria may occur during viral and febrile illnesses. This implies that there is different or additional pathogenesis of persistent, non-remitting, and relapsing nephrotic syndrome and MCD. Recent research has focused on the role of regulatory T-cell (Treg) dysfunction in MCD.
Garin et al. [4] examined urine soluble CTLA-4 levels in patients with MCD in relapse and remission, other glomerular disease, and control subjects. CTLA-4 is a protein secreted by Treg cells which binds to CD80 and therefore blocks the costimulatory activation of T cells. Although there was not a significant difference in urine sCTLA-4 levels between these groups, there was a significant decrease in the urinary ratio of sCD80/sCTLA-4 in patients with MCD in remission, pointing to a role of Treg dysfunction and relative CTLA-4 deficiency in suppression the continued activation of CD80 in MCD.
Araya et al. studied T cells in a small group of patients with MCD in relapse, MCD in remission, control patients, and MPGN [5]. They found that Treg suppression of T-effector cells was decreased in MCD patients in relapse versus MCD patients in remission and control patients. This was the first direct evidence of impaired Treg function in MCD. More recently, investigators have reported that nuclear factor-related kappa B, which is involved in chromatin remodeling, is upregulated in CD4+ T cells and B cells in relapsing MCD, suggesting that alterations in transcription factors of immunity may also play an important role [6].
LeBerre et al. used Buffalo/Mna rats, a model for idiopathic nephrotic syndrome with histological focal segmental glomerular sclerosis (FSGS) lesions, to explore the activity of T cells and the effects of different treatments [7]. Buffalo/Mna rats develop nephrotic syndrome with FSGS lesions that recurs after transplant with a normal kidney, implying the presence of a circulating permeability factor. The deoxyspergualin derivative LF15–0195 induced remission of proteinuria and improved the pathological features of Buffalo/Mna rats compared to untreated rats. There was a similar but less profound effect of this treatment on recurrent disease after transplant. A series of experiments showed that LF15–0195 decreases the quantity of renal monocytes and T cells, decreased levels of IL-10 and IL-13, significantly increased Treg cell quantity, and was associated with increased expression of Treg transcripts, including CTLA-4. Moreover, untreated Buffalo/Mna rats that received Treg cells from treated Buffalo/Mna rats demonstrated decreased proteinuria, although these results were not significant. This study demonstrated that augmentation or supplementation of Treg cell function, including an increase in CTLA-4 expression, induces remission of disease in the Buffalo/Mna model of idiopathic nephrotic syndrome.
Barrat et al. showed that a combination of vitamin D3 and dexamethasone led to in vitro differentiation of mouse naïve CD4 T cells into Treg cells [8], which provides another potential mechanism of the effect of glucocorticoids in SSNS.
Induction of Podocyte Proteins
Recent studies have investigated the role of podocyte proteins CD80 and angiopoietin-like protein 4 in response to damage from exogenous injury such as puromycin and endogenous circulating factors that result in the development of MCD-like phenotypes. CD80 (also known as B7–1) is a transmembrane protein that is present on antigen presenting cells (APCs). It is a costimulatory signal for T-cell activation. In 2004, Reiser et al. first showed that CD80 is also expressed in mouse podocytes after injection with lipopolysaccharide (LPS). LPS injection resulted in proteinuria which was CD80 dependent (the proteinuria did not occur in CD80 knockout mice injected with LPS) and T and B cell independent (the proteinuria did occur in SCID mice injected with LPS) [9]. The same study found that LPS interacts with cultured podocytes via Toll-like receptor 4 (TLR-4) and results in podocyte actin cytoskeleton reorganization. Lai et al. then demonstrated that IL-13 overexpression in rats produced a MCD-like phenotype (albuminuria, hypoalbuminemia, hypercholesterolemia, normal histology on light microscopy, and podocyte foot process effacement on EM) which also resulted in increased glomerular expression of CD80 [10]. In 2011, Shimada et al. demonstrated that polyIC (a TLR-3 ligand) induced human podocyte CD80 expression and podocyte injury [11], as well as increased NFκ(kappa)B and type 1 and 2 IFN activity. Both CD80 mRNA silencing and NFκ(kappa)B inhibition attenuated CD80 expression in the presence of polyIC, which also resulted in decreased evidence of podocyte injury. This model may explain the propensity of viral infections to induce MCD. This study also showed that incubation with dexamethasone attenuated the increase in CD80 activity and podocyte injury in the presence of polyIC, suggesting a mechanism of the beneficial effects of corticosteroid treatment in MCD.
The clinical utility of CD80 measurement in humans with MCD has been explored in two studies. In 2009, Garin et al. showed that there was an increased urinary excretion of soluble CD80 (sCD80) in children and adolescents with MCD in relapse compared to those with MCD in remission, patients with other glomerular disease, and control subjects [12]. Levels of serum sCD80 were not elevated in MCD compared to the other patient groups. The same group then measured urinary CD80 levels in 17 patients with MCD compared to 22 patients with FSGS and found an increased urinary CD80/creatinine ratio in MCD in relapse compared to MCD in remission or FSGS [12]. Thus, CD80 may be a useful biomarker in MCD.
A second protein which has been shown to play a role in steroid-sensitive nephrotic syndrome is angiopoietin-like-4 (Angptl4), a secreted glycoprotein which is a member of the angiopoietin-like protein family. Angptl4 is highly expressed in the liver and adipose tissue and previously had only been shown to have low-level renal expression as shown by Northern blot of whole kidney specimens [13]. Clement et al. demonstrated that Angptl4 expression is upregulated in the glomeruli of rats exposed to several models of podocyte injury, including puromycin [14]. This upregulation was specific to models of SSNS compared to those of membranous nephropathy (Passive Heymann Nephritis), mesangial injury (Thy1.1 nephritis), and collapsing FSGS (injection of rats with serum from patients with collapsing FSGS). A transgenic mouse model showed that upregulation of podocyte Angptl4 resulted in proteinuria, normal light microscopy, foot process effacement, and loss of glomerular basement membrane charge, similar to changes seen in MCD. Increased levels of Angptl4 produced by adipose tissue did not lead to proteinuria or glomerular changes, suggesting that Angptl4 does not act as circulating permeability factor. This study also highlighted important implications for treatment of SSNS. Firstly, glucocorticoids attenuated proteinuria and decreased Angptl4 expression in rats exposed to puromycin, implying a target of steroid therapy in this disease. Secondly, the glomerular Angptl4 was shown to be hyposialylated, and the addition of sialic acid precursors to the diet of NPHS2-Angptl4 transgenic rats improved proteinuria, leading to the consideration of a steroid-sparing therapy for SSNS.
Taken together, these studies show that circulating factors (PA, LPS, IL-13, and polyIC) induce increased expression of proteins such as CD80 and Angptl4 in podocytes, resulting a MCD-like disease in vitro and in vivo. The effects of glucocorticoids and sialic acid precursors on these molecular pathways and subsequent disease states point to a link between these therapies and the pathophysiology of SSNS.
In summary, the prevailing theory on the pathogenesis of MCD suggests that an initial insult in the form of exogenous or endogenous circulating factors induces podocyte expression of proteins such as CD80 and Angptl4, which in turn leads to podocyte actin cytoskeleton disorganization, foot process effacement, detachment from the GBM, and the nephrotic syndrome. Many cases spontaneously resolve. Those that do not resolve may have abnormalities in Treg cell function, and immunosuppressive therapy with glucocorticoids may mitigate disease activity by affecting these pathways (Fig. 2.2).
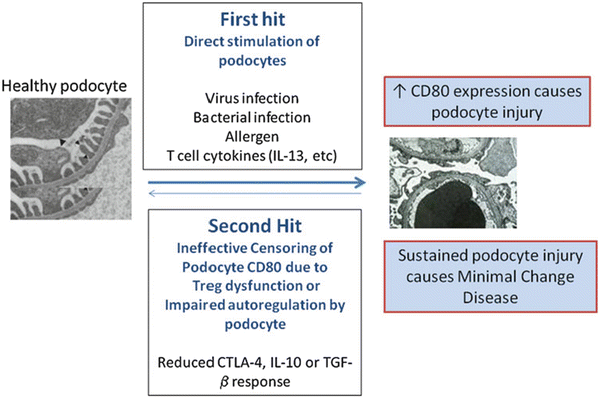

Fig. 2.2
Minimal change disease: a two-hit podocyte immune disorder. The first hit consists of the induction of CD80 in podocytes by microbial products, allergen, or T-cell cytokines such as interleukin (IL)-13. In normal settings, CD80 expression on podocytes is terminated by regulatory cytokines from T regulatory cells (Treg) and/or cytotoxic T lymphocyte-associated (CTLA)-4, and interleukin (IL)-10 by podocytes, and as a consequence, proteinuria is transient and mild. However, we propose a second hit occurs in MCD and consists of abnormal censoring of podocyte CD80 expression due to a defective autoregulatory response by Tregs or by the podocyte itself. As a consequence, CD80 expression becomes persistent and nephrotic syndrome results. From Shimada M, Araya C, Rivard C, Ishimoto T, Johnson RJ, and Garin EH. Minimal change disease: a “two-hit” podocyte immune disorder? Pediatr Nephrol. 2011;26(4):645–49. With kind permission from Springer Science and Business Media
Clinical Presentation
MCD classically presents with nephrotic syndrome characterized by heavy albuminuria, marked edema, and hypoalbuminemia with an accompanying elevation in circulating lipids. Spontaneous remission have been reported in MCD [15, 16], but because of significant morbidity including thromboembolism and infections in untreated patients [17, 18], most practitioners will elect to treat MCD patients. MCD in children is usually corticosteroid responsive with remissions occurring within a few weeks of starting in most cases. However, adults tend to respond less promptly and it may take up to 3–4 months after initiating steroid therapy for remission to occur. Also in contrast to children, up to 20–30 % of adults may fail to respond to steroid therapy, which implies different pathophysiologic mechanisms between the two age groups. A significant proportion of non-responders will show fibrosis on a subsequent biopsy with lesions of FSGS. Unlike in the pediatric age group, there is a paucity of well-designed randomized controlled trials (RCTs) in adult MCD.
MCD patients may present with acute kidney injury (seen in up to 20–25 % of adults) [19, 20]. Chronic kidney disease or end-stage kidney disease is not typically seen in adult MCD and should suggest other etiologies. MCD patients will typically experience relapses, and up to a third of patients may become frequent relapsers or corticosteroid-dependent [20–23]. Relapses are also seen in 40 % of adults who as children experienced MCD [24].
Secondary etiologies associated with MCD are uncommon but should be elucidated, especially in adults. They include malignancies (typically Hodgkin’s disease and thymoma), lithium therapy, and non-steroidal anti-inflammatory drugs [25].
From a risk perspective, severe nephrotic syndrome is associated with significant morbidity from dyslipidemia [26], infections [18, 27], and thromboembolic events [28]. These risks, along with the relative ease and tolerance of a short course of corticosteroids, prompt most physicians to treat patients with severe nephrotic syndrome. Drug-related adverse effects, however, are common with prolonged/repeated steroid courses in steroid-dependent (SD) or frequently relapsing (FR) patients.
Immunosuppressive/Immunomodulatory Treatment of MCD
Treatment of the Initial Episode
Corticosteroids
There are few controlled studies of immunosuppressive therapy in adults (Table 2.1) [15, 16, 29]. Experience with corticosteroids are mainly derived from large prospective RCTs in children [30, 31] and observational studies in children and adults [19, 20, 22, 23]. A multicenter controlled study of corticosteroids in 125 adult patients (including 31 MCD patients), which used at least 20 mg/day of prednisone for at least 6 months, showed an early and rapid decrease in proteinuria compared to the control group. However, by 2½ years, a significant number of patients in the control group underwent a spontaneous remission, leading ultimately to similar outcomes with respect to proteinuria or serum albumin in the two groups [15]. Similarly, in another randomized controlled trial which compared prednisone 125 mg every other day for 2 months with placebo in 28 adult MCD patients, there was no difference in overall remission rates over 77 months of follow-up. Similar to the previous study, patients remitted more rapidly when treated with prednisone, with 12 of 14 treated patients in complete remission before 2 months compared to 6 of 14 controls [16, 32].
Table 2.1
Controlled studies of MCD in adults
Reference | Study | N | Treatment protocol | Results |
|---|---|---|---|---|
Black et al. [15] | Multicenter controlled trial | 125 (31 with MCD) | prednisone 20–30 mg/day × >6 months (>10 mg/12 months) | Earlier response in treated patients |
Coggins [16] | Multicenter controlled trial | 28 | prednisone 120 mg every other day for 2 months | Earlier response in treated patients. No difference at 2 years |
Imbasciati et al. [29] | Multicenter (adults + children). Pulse IV steroids versus high dose oral prednisone | 22 adults (67 children) | Methylprednisolone 20 mg/kg/day × 3 days | Earlier response in children, no difference in adults. Tendency to earlier/frequent relapses in pulse. More side effects in oral group
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




