Preeclampsia
HELLP syndrome
Active lupus nephritis
Timing in pregnancy (weeks gestation)
After 20 weeks
After 20 weeks
Any gestational age
Complement (C3, C4)
Normal
Normal
May be decreased
Thrombocytopenia and neutropenia
Absent
Present
May be present
Active urine sediment or other organ involvement
Absent
Absent
Commonly present
Anti-dsDNA or Anti-C1q
Absent
Absent
May be present
Abnormal liver function tests
Absent
Present
Absent
Serum uric acid
Increased
Increased
Normala
Hypertension (>140/90 mmHg)
Present
Absent in 10–15 %
Variable
Serum creatinine >1.2 mg/dL
Typically absent
<10 % of patients
Commonly present
The ratio of complement activity (CH50) to complement split products (Ba) is preferred to measuring serum complement when differentiating a lupus flare from preeclampsia. In patients with a lupus flare, low CH50 levels are associated with elevations of complement split products, such as Ba, thus resulting in a lower ratio of CH50/Ba than in non-SLE patients with preeclampsia [18]. Newer biomarkers, including anti-angiogenic markers, such as soluble vascular endothelial receptor, 1 (sFlt-1) [19], may also be useful in making the distinction between a lupus flare and preeclampsia in the future. It is vital to distinguish LN (which will require immunosuppression) from preeclampsia, which is best managed with delivery.
If required, a renal biopsy can be performed safely in patients with optimal control of hypertension and stable coagulation parameters [20]. Of note, while early studies have reported high complication rates with renal biopsies in pregnancy, including perirenal hematoma (4.4 %) and gross hematuria (16.7 %) [21], more recent reports indicate complication rates that are similar to those in nonpregnant women [22, 23]. After 32 weeks of gestation, delivering the baby prior to contemplating a renal biopsy should be considered and discussed with obstetrical and neonatal providers.
LN Flare in Pregnancy
Studies to date have reported conflicting data [24–26] with respect to the likelihood of developing an LN flare during pregnancy. The most likely reason for these differing results is inconsistency in selecting appropriate controls, with some studies using nonpregnant controls and others using patients’ own nonpregnant disease courses [27–29]. The studies based on the latter type of design consistently have reported an increased likelihood of LN flare during pregnancy, with the risk approaching 70 % [30]. A meta-analysis of 37 studies of SLE pregnancies reported a lupus flare in approximately one in four pregnancies [31]. As activation of LN may lead to adverse maternal and fetal outcomes, close monitoring, with monthly assessments of disease activity in pregnant LN patients, is indicated (Table 22.2, Fig. 22.1). This approach may facilitate early detection and timely treatment of an LN flare, if and when it occurs.
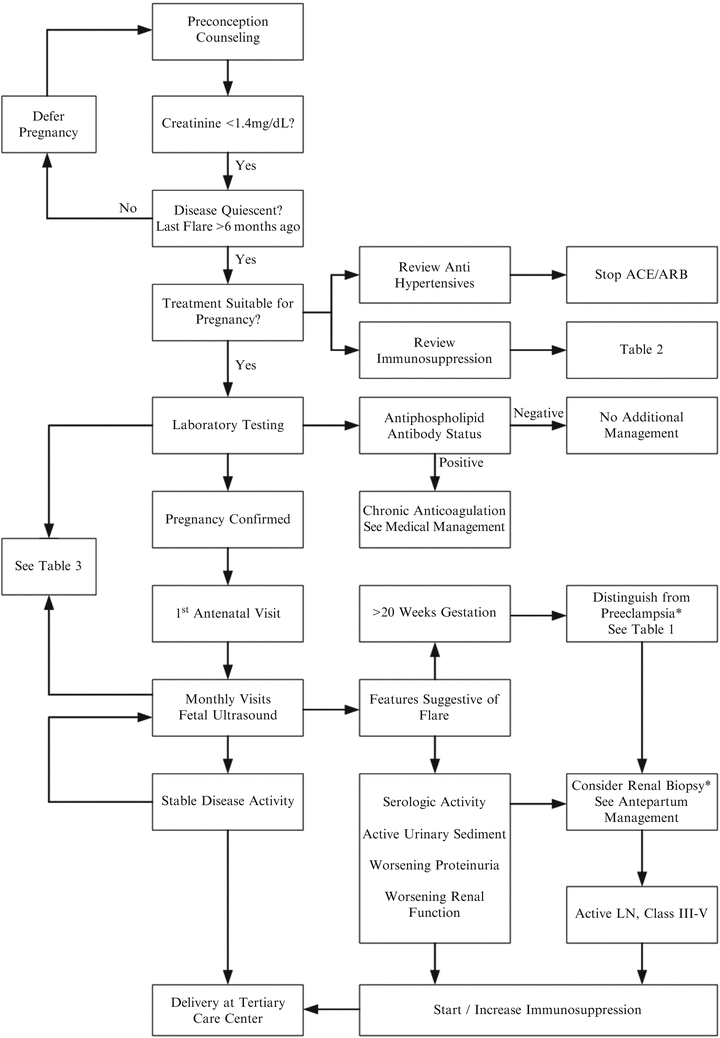
Table 22.2
Laboratory testing of patients with lupus nephritis during pregnancy
Timing | Suggested laboratory investigations |
|---|---|
Preconception counseling and/or first prenatal visit | •Urinalysis •Determination of proteinuria (optimally 24-h urine or protein/creatinine ratio) •Complete blood count •Serum creatinine •Antiphospholipid antibodies •Anti-SSA/Ro and Anti-SSB (La) antibodies: if positive, weekly fetal heart rate evaluation from weeks 16–24 and every other week from weeks 24–32 •Anti-double-stranded DNA antibodies •Complement studies •Liver function tests |
Every month | •Urinalysis and measurement of proteinuria •Serum creatinine •If any tests are abnormal, obtain lupus serologies, complement studies, and consider renal biopsy before 32 weeks |
Every trimestera | •Complete blood count •Anti-double-stranded DNA antibody •Complement studies •Liver function tests (for patients on azathioprine) |

Fig. 22.1
Flow chart for management of active lupus nephritis during pregnancy. Reprinted with permission from Stanhope TJ, White WM, Moder KM, Smyth A, Garovic VD. Obstetric Nephrology: Lupus and Lupus Nephritis in Pregnancy. Clinical Journal of the American Society of Nephrology, 2012;7(12):2089–99
Maternal Mortality
Maternal deaths are relatively rare events and reporting bias makes the true rate difficult to determine. We recently reviewed published evidence of pregnancy-related maternal deaths in women with SLE and LN; all 17 maternal deaths occurred in those with active disease, with disease activity/complications and opportunistic infections being the two major causes [32]. These data further support current recommendations for the avoidance of pregnancy until SLE manifestations are quiescent, with a renal remission of ≥6 months. This may decrease the complication rates due to disease activity and reduce the use of aggressive immunosuppression and its related complications, including maternal death due to opportunistic infections. The presented evidence further supports the importance of a planned pregnancy, expert monitoring, and judicious use of immunosuppressive therapies.
Preconception Counseling and Antepartum Monitoring
Prior to attempting to conceive, women with SLE/LN should undergo a detailed evaluation of their disease activity, and renal function (Table 22.2), and their immunosuppressive medications should be changed to include those that are most appropriate for pregnancy (Table 22.3). The need to modify and/or discontinue immunosuppressive agents during pregnancy may contribute to an increased risk for LN flare.
Table 22.3
Immunosuppressive medications and pregnancy
Comments | FDA classa | Breast-feedingb | |
|---|---|---|---|
Anti-inflammatory drugs | |||
Nonsteroidal anti-inflammatory drugs (NSAID) | Avoidance after 28 weeks gestation because of the effects of NSAID-related prostaglandin inhibition on the fetal cardiovascular system (closure of ductus arteriosus) | C | Usually compatible |
Immunosuppressive drugs | |||
Corticosteroids | Risks of use often outweighed by risk of underlying disease. Potential risks for orofacial clefts (3/1,000 births) and premature birth | C | Usually compatible |
Hydroxychloroquine | Considered safe in pregnancy at 200–400 mg/day. Discontinuation during pregnancy associated with increased risk of lupus flare. May use for maintenance or mild flares | Not assigned | Usually compatible |
Cyclosporine | Can be maintained in pregnancy at lowest effective dose. No significant increase in rate of congenital malformations | C | Not recommended |
Tacrolimus | Can be maintained in pregnancy at lowest effective dose. Potential risks of neonatal hyperkalemia and renal dysfunction | C | Not recommended |
Rituximab | Limited safety data. May alter fetal and neonatal B-cell development | C | Not recommended |
IVIG (gamma globulin) | Data are lacking, but may be helpful for lupus nephritis flare refractory to medical therapy | C | Compatible |
Azathioprine | May use for flare during pregnancy. Consider as alternative to mycophenolate. Avoid doses >1.5–2 mg/kg/day due to risk of suppressed neonatal hematopoiesis | D | Not recommended |
Mycophenolate mofetil | Contraindicated during pregnancy due to teratogenicity | D | Not recommended |
Cyclophosphamide | Useful when maternal disease is life threatening. High risk of fetal loss, but less pronounced in more recent studies | D | Not recommended |
Methotrexate | High risk of miscarriage and congenital abnormality. Treatment should be withdrawn 3 months before pregnancy | X | Not recommended |
Antihypertensive agents | |||
Centrally acting agents | |||
Methyldopa | Recommended for use, despite the risks of neuro-depression | B | Usually compatible |
Clonidine | As efficacious as methyldopa, but has unproven safety | C | Possible milk effects |
Beta-blockers | |||
Labetalol | Recommended for hypertensive urgency and may be associated with a risk of fetal bradycardia and neonatal hypoglycemia | C | Usually compatible |
Atenolol | Contraindicated due to risk of intrauterine growth restriction | D | Not recommended |
Calcium channel blockers | |||
Nifedipine | Recommended for use. With all calcium channel blockers, there is a risk of interactions with magnesium, resulting in profound hypotension | C | Usually compatible |
Direct vasodilators | |||
Nitroprusside | Very effective in severe hypertension, but associated with cyanide and thiocyanate toxicity | C | Insufficient data; Possible infant risk |
Hydralazine | Efficacious oral agent, but associated with maternal neuropathy, drug-induced lupus, and neonatal thrombocytopenia | C | Usually compatible |
Diuretics | |||
Thiazides | Useful in chronic hypertension, heart failure, and renal disease and may cause volume contraction and electrolyte abnormalities | B | Possible milk effects |
Spironolactone | Possible fetal antiandrogen effects | C | Usually compatible |
Angiotensin-converting enzyme (ACE)-inhibitors and angiotensin II receptor blockers (ARB) | Contraindicated in pregnancy. Known fetal side effects include skull hypoplasia, anuria, renal failure, oligohydramnios, craniofacial deformation, and hypoplastic lung development | D | Possible infant risk |
Antihypertensive antihypertensive medications, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) should be discontinued prior to pregnancy due to their known teratogenic effects [33] and replaced by a safe alternative(s) [34], such as methyldopa, labetalol, or nifedipine (Table 22.3). A baseline serology panel also should be obtained, which will facilitate the evaluation of disease activity during pregnancy.
Renal biopsy data should be reviewed during the counseling session, when available [35]. Patients with proliferative forms of LN (class III or IV) may need careful antenatal surveillance. An increased incidence of hypertension and preeclampsia has been reported in patients with class III or IV LN (37.1 %), compared with either class II or V (11.1 %) or age, parity, and duration of SLE-matched controls without renal involvement (11.6 %) [36]. During pregnancy, monthly prenatal visits, regular examinations (including serial fetal ultrasounds), and laboratory evaluations (Fig. 22.1, Table 22.2) are indicated.
Immunosuppression During Pregnancy
Immunosuppressive therapy may be required, for the first presentation of LN during the course of pregnancy, to maintain disease remission or to manage an LN flare during pregnancy. Both induction and maintenance regimens in pregnancy are based on corticosteroids, azathioprine, and calcineurin inhibitors (cyclosporine and tacrolimus) due to their acceptable safety profiles (Table 22.3), established mainly through their use in pregnant women with either inflammatory bowel disease or prior renal transplantation.
While previous studies have reported an association between corticosteroid use and orofacial clefts [37, 38], a more recent large-scale population study from Denmark did not confirm this association [39]. In addition, low-dose prednisone (5–10 mg/day) is unlikely to cause adrenal insufficiency and/or thymic hyperplasia in the fetus [40].
Azathioprine has been assigned to category “D” by the US Food and Drug Administration (FDA), mainly based on the data reporting increased risks for atrial and ventricular septal defects and preterm birth [41]. However, the results of a study of azathioprine use in pregnancies complicated by SLE reported favorable outcomes, supporting its use in pregnant patients with SLE or LN [42].
With respect to calcineurin inhibitors, cyclosporine is most commonly used due to its acceptable safety profile, which has been confirmed by both animal and human studies [43]. On the other hand, the use of tacrolimus over cyclosporine should be considered for induction, based on data suggesting that comparable rates of remission may be achieved with tacrolimus compared to cyclophosphamide [44]. While these data are insufficient to support the use of tacrolimus for induction in the general population, they may argue for its use in pregnant patients for whom mycophenolate mofetil and cyclophosphamide are contraindicated given their known teratogenic potentials [42, 43, 45, 46]. Optimally, mycophenolate mofetil should be discontinued 6 weeks prior to attempted conception [47].
In the postpartum period, caution is required, as a number of medications are secreted in breast milk and are not considered safe for infants (Table 22.3).
Antiphospholipid Antibodies/Syndrome
The presence of antiphospholipid antibodies, including lupus anticoagulant and anticardiolipin antibodies, in association with venous or arterial thromboses and/or pregnancy complications (such as recurrent miscarriages), constitute the diagnostic criteria of antiphospholipid syndrome (APS). Both the presence of antiphospholipid antibodies and APS may be seen as separate disease entities, or in association with SLE, with pregnancy complications including fetal loss, which generally occurs after 10 weeks of gestation, and an increased relative risk of preeclampsia [48, 49]. A meta-analysis reported the presence of antiphospholipid antibodies in about one quarter of SLE pregnancies [31]. Screening is recommended for the presence of these antibodies during the initial evaluation of pregnancies complicated by SLE or LN, as the risk of pregnancy loss has been correlated with the number of positive tests for the different antiphospholipid antibodies [50].
The mainstay of APS management is anticoagulation, while immunosuppression is reserved for those who, in addition to APS, have active SLE. Patients treated with warfarin before pregnancy should receive therapeutic anticoagulation, with either unfractionated heparin (UFH) or low molecular weight heparin (LMWH) while pregnant, as warfarin is contraindicated due to its teratogenic effects and the potential for life-threatening hemorrhage in the infant [51]. Pregnancy is a hypercoagulable state, and the risk for thrombotic events is further increased by the obstruction of venous return by the enlarged uterus. Therefore, for patients not treated before pregnancy, anticoagulation is indicated for all SLE patients positive for antiphospholipid antibodies and a history of thrombotic event(s), and for those without a previous history of thrombotic event(s), but meeting the obstetric criteria for APS, such as three or more pregnancy losses or a late pregnancy loss.
For women with antiphospholipid antibodies who do not meet the clinical criteria for APS, an acceptable approach is close clinical surveillance; other options include antepartum aspirin or prophylactic UFH or LMWH. Finally, even in the absence of antiphospholipid antibodies, prophylactic anticoagulation should be considered for pregnant LN patients with nephrotic syndrome, as the hypercoagulable state inherent to nephrotic syndrome may worsen further with pregnancy, leading to an increased risk for thromboembolic complications [52].
In general, for patients requiring anticoagulation during pregnancy, heparin should be started immediately after confirmation of an intrauterine pregnancy and continued for at least 6 weeks postpartum. Specific therapeutic and prophylactic anticoagulation regimens, therapeutic goals, and monitoring strategies are available in the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines: Venous Thromboembolism, Thrombophilia, Antithrombotic therapy, and Pregnancy [52].
Women with a history of antiphospholipid syndrome (APS) and arterial thrombotic events, stroke, in particular, should be advised against pregnancy due to the high risks for severe maternal complications, including death, which may sometimes occur despite chronic anticoagulation [53]. For these women, gestational carriers (surrogate mothers) can be considered for achieving parenting goals.
Systemic Sclerosis/Scleroderma
Scleroderma is more prevalent in women and has a mean age of onset of 40 years [54]. As a result, it is not commonly encountered in women of childbearing age. It is unclear if pregnancy increases the risk of scleroderma renal crisis, which presents with abrupt renin-mediated hypertension and renal impairment. Optimal treatment is with ACE inhibitors or ARBs [55], which poses a major challenge in pregnant patients, as these agents are generally considered to be contraindicated for use due to the risk of congenital malformations (Table 22.3). However, in the setting of life-threatening maternal disease, their use in pregnancy may be justified. Renal crisis affects 5 % of patients with systemic sclerosis and most commonly presents within the first 5 years of disease onset [56]. Therefore, women diagnosed with scleroderma should be counseled that delaying pregnancy may reduce the risk of renal crisis. Patients with CREST (calcinosis, Raynaud’s, esophageal dysmotility, sclerodactyly, telangiectasia) syndrome, a limited form of scleroderma, typically do not have renal involvement and usually have good pregnancy outcomes.
IgA Nephropathy
Immunoglobulin A (IgA) nephropathy, the most prevalent primary glomerulonephritis, is most commonly diagnosed in the second and third decades and affects many women of childbearing age. It is uncertain if pregnancy adversely affects long-term renal outcomes, as most studies have included heterogenous populations and report inconsistent findings. A recent study reported that pregnancy does not affect long-term renal outcomes in patients with IgA nephropathy and near-normal kidney function [57]. Another reported that strategies resulting in the reduction of proteinuria prepregnancy were associated with preservation of postpartum renal function [58]. The risk of worsening renal disease during pregnancy is highest when the baseline GFR is <70/mL/min, and either the presence of uncontrolled hypertension or severe arteriolar and tubulointerstitial disease on renal biopsy [59].
The majority of agents used in the of management of IgA nephropathy are nonimmunosuppressive and include ACE inhibitors or ARBs, both of which are contraindicated during pregnancy (Table 22.3). Immunosuppressive agents are increasingly used for the treatment of IgA nephropathy, with clinical (hematuria, rising serum creatinine and rising proteinuria, despite anti-proteinuric therapy) and histologic evidence (necrotizing glomerular lesions) of active inflammatory changes. Most patients with mild, stable, or slowly progressive IgA nephropathy are not treated with immunosuppression. In general, ACE inhibitors should be discontinued prior to pregnancy and immunosuppressive agents should be reviewed and changed to pregnancy-safe alternatives (Table 22.3), ideally prior to conception or at the earliest indication of pregnancy in order to minimize fetal risks.
Diabetic Nephropathy
Diabetic nephropathy, a progressive disease characterized by proteinuria, hypertension, and reduced GFR, is present in approximately 6 % of pregnant women with type 1 diabetes [60]. As the majority of patients with type 2 diabetes are older when diagnosed, typically they have a lower prevalence of diabetic nephropathy during their childbearing years. The mainstay of management is strict glycemic control, which has resulted in improved perinatal survival to 95 % [61]. The risk of pregnancy complications is correlated with the degree of prepregnancy renal impairment [62].
Women with diabetic nephropathy are at an increased risk for preeclampsia, regardless of the severity of proteinuria in early pregnancy [63]. The risk for deterioration in renal function and/or progression to ESRD during or after pregnancy is highest with a baseline serum creatinine measurement of ≥1.4 mg/dL [61]. Ideally, these women should undergo preconception evaluation and counseling as to the potential risks associated with pregnancy.
ACE inhibitors or ARB should be changed to agents safer for use during pregnancy to maintain control of hypertension (Table 22.3). Studies have shown that prior treatment with an ACE inhibitor, in combination with intensive glycemic control for 3–6 months prior to conception, is associated with a renal protective effect during pregnancy [64].
Focal Segmental Glomerulosclerosis
Focal segmental glomerulosclerosis (FSGS) is one of the most common causes of nephrotic syndrome [65] and is the most common primary glomerular disorder that causes ESRD in the USA [66]. FSGS may present in association with IgA nephropathy, vasculitis, lupus nephritis, infection (e.g., HIV, parvovirus, cytomegalovirus, Epstein-Barr virus), or drugs/toxins (e.g., interferon, heroin, lithium, pamidronate). Primary FSGS, accounting for approximately 80 % of cases of FSGS [67], typically presents with acute or subacute onset of nephrotic syndrome (peripheral edema and hypoalbuminemia), in contrast to secondary FSGS, which usually presents with slowly progressive non-nephrotic range proteinuria. Renal insufficiency at presentation is more common in cases of secondary FSGS.
In pregnancy, it is important to distinguish FSGS from preeclampsia, as FSGS may require immunosuppression and preeclampsia is best treated with delivery. As previously mentioned, a renal biopsy can be performed safely during pregnancy. Renal biopsies from patients with preeclampsia may show features similar to FSGS [68], and biopsy findings should be correlated within the clinical context in order to make a definitive diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







