Essentials of Diagnosis
- The clinical features of nephrotic syndrome are present.
- The glomeruli are not inflamed.
- The glomerular capillary basement membrane appears thickened.
- There is an absence of glomerular inflammation.
- Idiopathic membranous nephropathy (MN) is relatively common and remains the leading cause of nephrotic syndrome in white adults. It is diagnosed by ruling out secondary causes.
- Secondary MN forms may account for up to one-third of cases and are associated with autoimmune diseases.
- Malignancy increases with increasing age.
General Considerations
Sixty years ago E.T. Bell coined the term membranous glomerulonephritis to describe the renal pathology of a group of patients with the clinical features of nephrotic syndrome in whom the glomeruli were not inflamed but in whom the glomerular capillary basement membrane appeared thickened. The synonymous terms extramembranous nephropathy and epimembranous glomerulonephritis have also been used to describe the disease. The word glomerulonephritis is, however, misleading as one of the main features of the condition is the absence of glomerular inflammation; because of this the word nephropathy is more often employed.
Idiopathic MN is a relatively common immune-mediated glomerular disease and remains the leading cause of nephrotic syndrome in white adults. In the majority of cases, the etiologic agent is unknown, and the disorder is termed idiopathic. Secondary MN forms may account for up to one-third of cases and are associated with autoimmune diseases (eg, systemic lupus erythematosus, SLE), infections (eg, hepatitis B and C), medications [eg, nonsteroidal antiinflammatory drugs (NSAIDs), d–penicillamine, gold], and neoplasias (eg, colon cancer, kidney cancer) (Table 26–1). The association with malignancy increases with age, reaching up to 20% in patients over the age of 60 years. Since idiopathic and secondary forms have similar clinical presentations, the designation of idiopathic is made by ruling out secondary causes by a careful history, physical examination, and laboratory evaluation of the patient. The disease is rare in children, and when it does occur, is commonly associated with an immunologically mediated disorder such as SLE.
Infections: Hepatitis B and C,1 syphilis (congenital and secondary), leprosy, filariasis, hydatid cyst disease, hepatosplenic schistosomiasis, echinococcus, post-streptococcus infection, malaria |
Neoplasias: Carcinomas,1 leukemia, lymphoma, pheochromocytoma, carotid body tumor |
Autoimmune: SLE,1 thyroiditis, rheumatoid arthritis, mixed connective tissue disease, sarcoidosis, angiolymphoid hyperplasia with eosinophilia (Kimura disease), primary biliary cirrhosis, Sjögren’s syndrome, ankylosing spondylitis, dermatitis herpetiformis |
Drugs: NSAIDs (diclofenac1), gold, d–penicillamine, mercury, captopril, formaldehyde, thiola, probenecid, bucillamine, tiopronin |
Other: de novo renal transplant, sickle-cell disease, Gardner–Diamond syndrome, Guillain–Barré syndrome, graft-versus-host disease following bone marrow transplant, diabetes mellitus |
Pathogenesis
There is considerable evidence to support the hypothesis that idiopathic MN is an autoimmune disease. However, the pathogenic mechanisms that cause immune deposits of immunoglobulin G (IgG) and complement to localize predominantly or exclusively on the subepithelial surface of the glomerular basement membrane (GBM) and the subsequent development of proteinuria are not fully understood. The Heymann model of experimental MN in rats, which closely mimics the histologic features of human disease, suggests that the podocyte is the target of injury. The antigenic targets of the antibody response in this experimental model have been localized to the clathrin-coated pits of the podocyte foot processes. They are, specifically, a 515-kDa glycoprotein called megalin, a polyspecific receptor related to the low-density lipoprotein receptor family that functions as a multiligand receptor for the uptake of a variety of macromolecules (eg, aminoglycosides, advanced glycation end products, vitamin D) and a second 44-kDa protein, know as RAP (receptor-associated protein), that is closely related to megalin and is an additional target antigen in the model. Binding of the antibody to the antigen results in activation of the complement with insertion of C5b-9 (membrane attack complex) to the podocyte membrane. Because the antigen–antibody binding and subsequent complement cascade activation occurs on the urinary side of the GBM, and thus away from the circulation, the cellular inflammatory response is blunted, and this accounts for the absence of inflammatory cells in the glomeruli on light microscopy. Although the insertion of C5b-9 to the podocyte membrane is insufficient to cause cell lysis, it does induce podocyte activation and signal transduction, resulting in an increased production of a number of potentially cytotoxic molecules (eg, cytokines, toxic oxygen radicals, proteases, vasoactive substances) that will ultimately damage the underlying GBM. The result is excessive synthesis of GBM, increased glomerular permeability, development of nonselective proteinuria, and effacement of the foot processes. The reduction in the glomerular filtration rate (GFR) results from progressive thickening of the GBM and reduced glomerular hydraulic permeability coefficient (Kf) whereas the effects of proteinuria on tubular cell differentiation and cytokine release engender progressive tubulointerstitial fibrosis. Megalin, however, is not present in human glomeruli, and except for rare cases secondary to hepatitis B or thyroiditis, where the specific antigen has been found as part of the immune complex, and a well-documented case of congenital MN due to passive transfer of a maternal antibody against a neutral endopeptidase present on the infant’s glomerular podocyte, the nature of the antigen involved in the majority of cases of idiopathic MN remains unknown.
It is quite likely that a number of different antigen-antibody combinations may cause MN. Some antigens may be endogenous, while others may be exogenous. The electrical charge of the antigens, antibodies, and/or circulating immune complexes, as well as their size, may play a role in determining whether an immune complex will form in a subepithelial position. There is an increased risk for MN in white patients with HLA-DR3 and in Japanese patients with HLA-DR2. The rare occurrence of MN in more than one family member suggests that genetic factors may be involved in the pathogenesis related either to a predisposition for developing the disease or its progression.
Pathogenesis
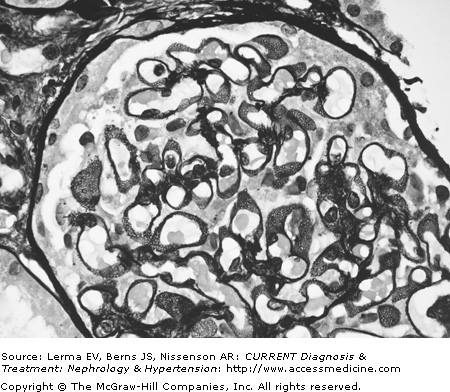
The diagnosis of MN is made by renal biopsy. In very early cases of MN, the glomeruli appear normal by light microscopy (hematoxylin and eosin), although abnormalities may be seen in silver preparations and by immunofluorescence and electron microscopy. Capillary loops are widely patent and the glomeruli show no increase in cellularity and there is no nuclear crowding. As the number and size of subepithelial immune complexes increase, the GBM develops a diffuse and uniform thickening on light microscopy. These changes affect all the glomeruli. Thin sections examined by the periodic-silver-methenamine stain demonstrate the classical “spike” pattern on the epithelial side of the basement membrane reflecting the increased synthesis and deposition of GBM-like material around the immune deposits (Figure 26–1). As the disease progresses, thickening of the capillary wall becomes pronounced, the capillary lumen narrows, and eventually sclerosis and hyalinization of the glomerular tuft develop.
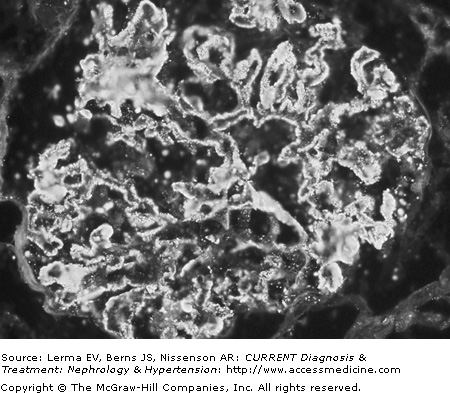
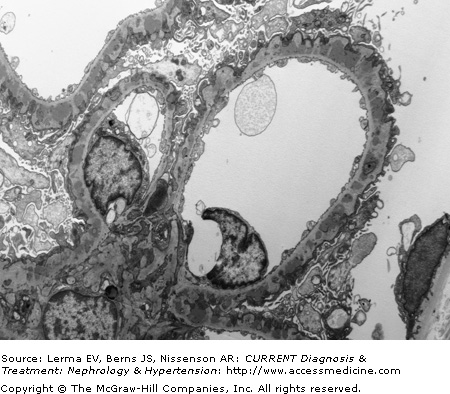
Proximal tubules are remarkable for the lipid vacuoles in the cytoplasm and numerous proteinaceous casts in the lumen. In the initial stages the interstitium is often normal, but with progression of the disease fibrosis and lymphocyte infiltrates are present. Immunofluorescence microscopy shows a very characteristic and uniform deposition of IgG and C3 in a granular pattern along the epithelial side of the GBM (Figure 26–2). IgG (with IgG4 being the predominant IgG subclass) is present in >95% of cases, but C3 may be seen in only 30–50% of cases of idiopathic MN. It has been suggested that positive C3 staining represents active, ongoing immune deposit formation and complement activation at the time biopsy was performed, whereas the absence of C3 reflects cessation of the immunopathologic process. Electron microscopy demonstrates that the typical electron-dense deposits are localized in the subepithelial space together with effacement of the foot processes (Figure 26–3). Deposits usually have a synchronous, homogeneous electron-dense appearance, but a heterogeneous type with dense deposits at various stages of formation can also be found. A four-stage (I–IV; Table 26–2) classification system has been developed based on their specific localization. Unfortunately the clinical and laboratory correlation with these stages is poor.
Rare in children: <5% of total cases of NS |
Common in adults: 15–50% of total cases of NS, depending on age; increasing frequency after age 40 years |
Males > females in all adults groups |
Whites > Asians > African-Americans > Hispanics |
NS in 60–70% |
Normal or mildly elevated BP at presentation |
“Benign” urinary sediment |
Nonselective proteinuria |
Tendency to thromboembolic disease (DVT, RVT, PE) |
Secondary forms on MN have histologic features similar to idiopathic MN. However, the presence of deposits of immunoglobulins other then IgG (ie, IgA and IgM), particularly in the mesangium, small subendothelial deposits, tubular basement membrane deposits, and intense C1q deposition are more suggestive of membranous nephropathy secondary to SLE, hepatitis B, or drugs (gold, d–penicillamine).
Clinical Findings
The disease affects patients of all ages and races, but is more common in men than women by about a 3:1 ratio. Idiopathic MN is most often diagnosed in middle age, with the peak incidence during the fourth and fifth decades of life, and is relatively uncommon in patients under 20 years. At presentation 60–70% of patients will have the nephrotic syndrome (NS) and its associated features: edema, hypoalbuminemia, and hyperlipidemia. The remaining 30–40% of patients present with subnephrotic range proteinuria (<3.5 g/24 hours), most commonly found at the time of routine examination in an otherwise asymptomatic patient. Proteinuria is nonselective. The presence of microscopic hematuria is common (30–40%), but macroscopic hematuria and red cell casts are rare and suggest a different histopathology. In patients with idiopathic MN serum C3 and C4 complement levels are always normal. At the time of diagnosis, the majority of patients are normotensive; only 10–20% are hypertensive. Renal function is normal in the majority of patients at presentation, with only a small fraction (<10%) exhibiting renal insufficiency (Table 26–3) Additional complications related to the disease include a variety of abnormalities in the lipid profile, which probably contribute to the increased cardiovascular risk seen in these patients, and a high prevalence of thromboembolic events including renal vein thrombosis in 10–40% of patients.
Stage I or early stage: Light microscopy shows a normal glomeruli or slightly thickened capillary walls. There is no evidence of “spike-like” projections or only very scattered. Few and small, superficially placed, subepithelial electron-dense deposits on EM. Fusion of foot processes in the region of the deposits. |
Stage II or fully developed lesion: Diffuse, uniform thickening of capillary walls on light microscopy. Prominent, “spike-like” projections along the GBM. Numerous, larger, and more confluent deposits cover the entire capillary loop on EM. |
Stage III or advanced lesion: Highly irregular and thickened capillary walls (“moth-eaten” appearance). EM shows deposits (electron dense and lucent) have been encircled by the GBM (“domes”) and become intramembranous. |
Stage IV or late stage: Deposits become more lucent or absent, and fewer in numbers with numerous electron-lucent vacuolated areas seen within a markedly thickened GBM (“swiss cheese”). Glomerular collapse and fibrosis are found on light microscopy. |
Differential Diagnosis
The differential diagnosis includes other causes of NS such as minimal change disease, focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis type I and II, amyloidosis, light chain deposition disease, and diabetic nephropathy. It is important to exclude secondary causes of MN, particularly, hepatitis B, SLE, malignancy, and drugs. In patients less than 16 years of age secondary MN is most commonly due to viral infection or SLE, while in adults >60 years, secondary MN is usually due to malignancy or drugs (Table 26–4). In patients with MN, ruling out secondary causes, apart from a thorough history and physical examination, should involve appropriate laboratory evaluation including complement profile, hepatitis serology, antinuclear antibodies, a chest x-ray, testing for occult blood in the stools, a mammogram in women, and prostate-specific antigen testing in men.
In a biopsy series from patients with SLE, MN histology accounts for 8–27% of cases of lupus nephritis. Patients with “pure” membranous lupus nephropathy often have no clinical symptoms that could suggest SLE and serologic markers of lupus activity, such as serum complement and anti-dsDNA levels, are frequently normal and do not correlate with disease activity. Hepatitis B-associated MN occurs frequently in hepatitis B- prevalent areas of the world and affects both adults and children who are chronic carriers of hepatitis B virus [positive hepatitis B surface antigen (HbsAg), hepatitis B core antigen (HbcAg), and usually hepatitis B early antigen (HBeAg)], with or without a history of overt liver disease. In children with hepatitis B-associated MN the nephrotic syndrome usually has a benign course, but progressive renal insufficiency is common in adults.
Hypocomplementemia is present in approximately 50% of the reported cases. Solid tumors (eg, carcinoma of the lung, colon, or kidney) are the most common underlying malignancy involved in cases of tumor-induced MN. It is hypothesized that antigen(s) derived from the tumor are deposited in the glomeruli where they trigger an antibody response and activation of the complement cascade, leading to disruption of the GBM integrity and podocyte injury. In some patients proteinuria resolves with removal or adequate treatment of the tumor. There are, however, well-described cases in which no improvement or remission of the proteinuria occurred following removal of the tumor.
In cases of MN secondary to drugs, discontinuation of the offending agent usually results in complete remission of the nephrotic syndrome. Although resolution of the proteinuria may occur as early as 1 week following discontinuation of the offending drug (eg, NSAIDs), it is not unusual for proteinuria to persist and in certain types associated with the use of gold or d–penicillamine it may take up to 3 years for remission of the proteinuria to occur (mean: 9–12 months). A number of glomerular pathologies have been reported to occur in association with or superimposed upon MN. Such diseases include IgA nephropathy, focal and segmental glomerulosclerosis, crescentic glomerulonephritis (anti-GBM disease, ANCA vasculitis), acute interstitial nephritis, and diabetic nephropathy. In some diabetic patients, MN is thought to occur as a consequence of the development of antiporcine insulin antibodies against porcine insulin deposited along the GBM.