Phuong-Thu Pham, Son Pham, Phuong-Anh Pham, Gabriel M. Danovitch
Medical Management of the Kidney Transplant Recipient
Cardiovascular Disease and Other Issues
The medical management of transplant-related complications is discussed in this chapter; post-transplant infections, gastrointestinal problems, and malignant neoplasms are discussed in Chapter 105.
Cardiovascular Disease
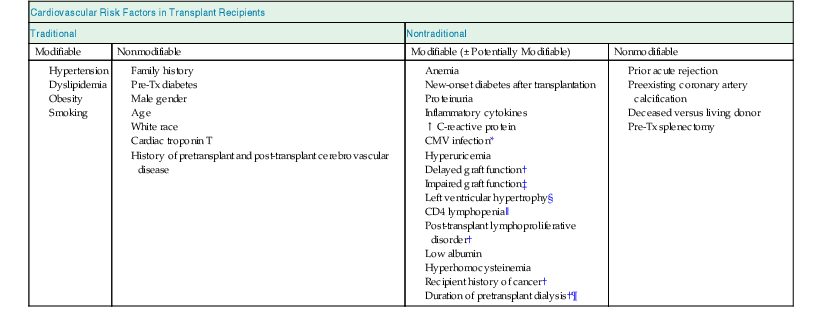
Cardiovascular (CV) disease is the most frequent cause of death with a functioning graft. In a large multicenter, international study consisting of more than 23,000 kidney transplant recipients, the cumulative incidences of coronary heart disease (CHD) at 1, 3, and 5 years after transplant were 3.1%, 5.2%, and 7.6%, respectively. Of nearly 700 CHD events that occurred in the first post-transplant year, 49% were nonfatal acute myocardial infarction (AMI) and 13% fatal AMI or sudden death.1 Renal function, estimated glomerular filtrate rate (eGFR), and preexisting CV disease were found to be the most important risk factors for a CV event. Recently, an analysis based on the Assessment of Lescol in Renal Transplantation (ALERT) trial suggested that major CV events could be predicted with a seven-variable model including age, previous CHD, diabetes, low-density lipoprotein (LDL), creatinine, number of transplants, and smoking.1a Although kidney transplantation ameliorates some CV risks by restoring renal function, it introduces new CV risks, including impaired glucose tolerance or diabetes mellitus, hypertension, and dyslipidemia, which are derived in part from immunosuppressive medications. Kidney transplant recipients have both conventional and unconventional CV risk factors (Table 106-1).2
Table 106-1
Cardiovascular risk factors in transplant recipients.
CMV, Cytomegalovirus; CNI, calcineurin inhibitor; Pre-Tx, pretransplant.
| Cardiovascular Risk Factors in Transplant Recipients | |||
| Traditional | Nontraditional | ||
| Modifiable | Nonmodifiable | Modifiable (± Potentially Modifiable) | Nonmodifiable |
* Strict adherence to CMV prophylaxis protocol and CMV surveillance in high-risk candidates.
‡ CNI minimization at the discretion of the clinicians.
§ Optimize blood pressure control.
‖ Assess risks and benefits of T cell–depleting antibody treatment (limited data).
¶ Discuss living donor kidney transplant option with patient at the time of transplantation evaluation.
Conventional Cardiovascular Disease Risk Factors
Post-transplantation Hypertension
Hypertension is a risk factor for both CV disease and kidney graft failure, with a graded risk for graft failure with increasing levels of systolic and diastolic blood pressure.3 Whether aggressive lowering of blood pressure retards the progression of graft failure beyond that of blood pressure control alone remains to be studied. Hypertension is common after transplantation and is present in 50% to 90% of kidney transplant recipients. Systolic blood pressure is highest immediately after transplantation and declines during the first year. In a Collaborative Transplant Study (CTS) registry, only 8% of transplant recipients had a systolic blood pressure below 120 mm Hg at 1 year; 33% had a blood pressure in the prehypertension range; 39% had stage 1 hypertension; and 20% had stage 2 hypertension despite antihypertensive therapy.3 Risk factors for post-transplant hypertension include preexisting hypertension, cyclosporine (and to a lesser degree tacrolimus), corticosteroids, poor quality of donor organ, delayed graft function, chronic allograft injury, high body mass index (BMI) or excess weight gain, acute rejection episodes, recurrent or de novo glomerulonephritis (GN), and transplant renal artery stenosis. Sodium intake after transplantation may also be contributory in salt-sensitive hypertensive patients. In rare patients, excess renin output from the native kidneys may contribute to post-transplantation hypertension. In kidney transplant recipients with severe hypertension refractory to medical therapy, bilateral native nephrectomy has been reported to ameliorate blood pressure control.4 Recently, renal artery nerve denervation has been reported to have benefit in nontransplant patients with resistant hypertension (see Chapter 38).5 Whether native kidney sympathetic denervation procedures improve blood pressure control in kidney transplant recipients with refractory hypertension remains to be studied.
Management of post-transplantation hypertension should include identification and treatment of the underlying cause, lifestyle modifications (see Chapter 35), and treatment of associated CV risk factors. The initial target blood pressure goal is below 130/80 mm Hg, and in patients with proteinuria, below 125/75 mm Hg. Although JNC 8 blood pressure targets for diabetics and patients with chronic kidney disease are set at higher levels than those of JNC 7, whether patients with post-transplant hypertension benefit from higher blood pressure goals remains to be studied. Although there is a lack of controlled clinical trials related to selection of antihypertensive agent, we recommend β-blockers in the perioperative setting because they have been shown to reduce CHD events in high-risk candidates. In the early post-transplantation period, nondihydropyridine calcium channel blockers (CCBs) and diuretics are frequently used, the former for their beneficial effect on intraglomerular hemodynamics and the latter to eliminate salt and water in patients who are volume expanded postoperatively. Although one retrospective cohort study reported an unexpected association between the use of dihydropyridine CCBs and an increased risk for CHD,6 results of another trial showed no significant association between any CV medication use (β-blockers, angiotensin-converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARBs], diuretics, CCBs, antiplatelet agents, and/or statins) and increased major cardiac event risks at 12 months.7 Studies evaluating the effect of CCBs on renal function have similarly yielded mixed and even contradictory results. In proteinuric chronic kidney disease (CKD) patients, dihydropyridine CCB use was shown to be associated with increased risk of renal disease progression and death except when used in conjunction with angiotensin II blockade therapy. However, results of a meta-analysis of randomized controlled trials demonstrated that CCB improved allograft function and reduced graft loss.8 The study findings suggest that treating 100 patients with average risk of graft loss over the first year (approximately 10%) with CCBs, compared with no treatment, would prevent three patients from losing their grafts, irrespective of the presence or absence of hypertension. However, CV disease events and mortality were not reported. Based on currently existing literature, there appears to be no contraindication to the use of CCs in the transplant setting, although monotherapy with dihydropyridine CCBs should be used with caution (see also Chapter 80).
Angiotensin-converting enzyme inhibitors and ARBs block calcineurin inhibitor (CNI) toxicity in experimental models and are protective in patients with CKD. However, they can cause acute changes in renal function as well as hyperkalemia and hence are usually not started until allograft function is stable. A meta-analysis of 21 randomized controlled trials demonstrated that ACE inhibitors and ARBs reduced proteinuria in transplant recipients, but they also lowered hematocrit by 3.5% and glomerular filtration rate (GFR) by 6 ml/min; there were insufficient data to determine the effect of ACE inhibitor or ARB use on patient or graft survival.9 Serum potassium and creatinine concentrations must be closely monitored; if serum creatinine rises more than 30% above baseline, then transplant renal artery stenosis should be considered.
Because of the lack of conclusive evidence that one class of antihypertensive agent is superior to another in the transplant setting, treatment should be individualized. Potential advantages and disadvantages of different classes of antihypertensive agents in kidney transplant recipients are shown in Table 106-2.
Table 106-2
Potential advantages and disadvantages of different classes of antihypertensive agents.
ACE, Angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CNI, calcineurin inhibitor.
| Potential Advantages and Disadvantages of Different Classes of Antihypertensive Agents* | ||
| Classes of Drugs | Advantages | Disadvantages |
| β-Blockers | Perioperative use: ↓ coronary heart disease events | ↑ Risk of bradycardia when used with nondihydropyridine calcium channel blocker Blunting of hypoglycemic awareness |
| Calcium channel blockers | ↓ CNI-induced renal vasconstriction† ↑ CNI level (may permit CNI dose reduction by up to 40%)‡ | Monotherapy with dihydropyridine calcium channel blocker should be used with caution§ |
| Diuretics | Beneficial in patients who are volume expanded | Hyperuricemia, gout |
| ACE inhibitors, ARBs | ↓ Proteinuria Potential renal protective and cardioprotective effects Beneficial in patients with post-transplantation erythrocytosis | Potential worsening of anemia |
| Aldosterone receptor blockers | May improve outcomes in heart failure | Severe hyperkalemia when used in combination with ACE inhibitor or ARB in patients with poor kidney function |
| α2-Blockers | Benign prostatic hypertrophy Neurogenic bladder | |
| Direct vasodilators | Tachycardia | |
| Central α-agonist | Depression | |
* In general, there is no absolute contraindication to the use of any antihypertensive agent in renal transplant recipients.
† Both dihydropyridine and nondihydropyridine CCBs.
‡ Nondihydropyridine CCBs.
§ See text.
Post-transplantation Dyslipidemia
Dyslipidemia is common after transplantation, in part because of the hyperlipemic effect of corticosteroids, cyclosporine, tacrolimus, sirolimus, and everolimus. Sirolimus and everolimus are associated with the worst lipid profiles, followed by cyclosporine, and to a lesser extent tacrolimus. One single-center study demonstrated that the mean values of total cholesterol, LDL cholesterol, and triglycerides (TGs) and the incidence of CHD were higher among patients receiving mammalian target of rapamycin (mTOR) inhibitors compared with their CNI-treated counterparts (control group). However, the risk of CV events was not significantly higher among patients receiving mTOR inhibitors compared with controls at 4-year follow-up.10 It is hypothesized that the antiproliferative effects of mTOR inhibitors may offset the adverse effects of hyperlipidemia. The dyslipidemic effect of mTOR inhibitors on the risk for CV disease events after kidney transplantation remains unclear; however, mTOR inhibitor–induced dyslipidemia should be treated aggressively. Other potential etiologic factors for post-transplant dyslipidemia include age, diet, rapid weight gain, hyperinsulinemia, preexisting hypercholesterolemia, allograft dysfunction, proteinuria, genetic predisposition, and the use of β-blockers and diuretics.
Although hyperlipidemia often improves within the first 6 months after transplantation as the doses of immunosuppressive agents are reduced, total and LDL cholesterol goals as defined by the National Cholesterol Education Program (NCEP) guidelines (www.nhlbi.nih.gov/about/ncep/index.htm) are usually not achieved. Hence, management of hyperlipidemia is usually required, and includes lifestyle changes (diet and exercise) and statins. In addition to their lipid-lowering effect, statins may offer protection against CV disease via their antiproliferative and anti-inflammatory properties and ability to reduce circulating endothelin-1, C-reactive protein levels, systolic and diastolic blood pressure, and pulse pressure.
The clinical benefits of statins in the general population have been demonstrated in several large randomized controlled trials. To date, there has been only one single prospective randomized trial in transplant recipients comparing statins (fluvastatin) with placebo—the ALERT study. The ALERT study showed that treatment of kidney transplant recipients with fluvastatin over a 5- to 6-year period significantly and safely reduced LDL cholesterol levels. The incidence of major adverse cardiac events was also shown to be reduced, albeit not statistically significantly. However, further analysis demonstrated a beneficial effect of early initiation of fluvastatin on outcome—the earlier the initiation of therapy, the greater the reduction in cardiac events. Patients who received statin therapy within the first 4 year after transplantation had a risk reduction of 64% compared with 19% for those who received therapy after 10 years. No statin effect on graft loss or doubling of serum creatinine was observed.11–12 Despite the well-established efficacy and safety of the use of statins in transplant recipients, clinicians should be aware that the use of statins with CNIs, particularly cyclosporine, often results in a several-fold increase in statin blood level and an increased risk for myopathy and rhabdomyolysis.13 Compared with other statins, simvastatin appears to be particularly prone to drug-drug interactions because of its extensive metabolism by CYP3A4.
Ezetimibe and statin combination therapy can significantly improve cholesterol control because of their complementary mechanisms of action. Ezetimibe blocks intestinal absorption of dietary cholesterol, whereas statin inhibits hepatic cholesterol synthesis. Ezetimibe used alone or as adjunctive therapy with statin appears safe and effective in the treatment of dyslipidemia in kidney transplant patients who are refractory to statin therapy. In a single-center study consisting of 67 patients with post-transplantation hyperlipidemia resistant to statins, treatment with ezetimibe alone or with ezetimibe and statin significantly reduced total cholesterol and LDL cholesterol by 25% and 34%, respectively, during the first month of treatment.14 In another study of 56 kidney transplant recipients, the addition of ezetimibe to statin therapy slowed the decline in renal function compared with controls.15 Whether ezetimibe-statin combination therapy is superior to statin-alone therapy in CV disease risk factor reduction remains to be elucidated.
Severe hypertriglyceridemia (TG level >500 mg/dl or 5.65 mmol/l) may occur with the use of sirolimus and everolimus. Management includes dose reduction, addition of a fibric acid derivative or nicotinic acid, and, in refractory cases, switching of sirolimus or everolimus to mycophenolate mofetil (MMF) or tacrolimus. The National Kidney Foundation expert panel recommends gemfibrozil as the fibrate of choice. However, fibrates should be avoided in those with CKD stage 5. Of the major fibric acid medications (bezafibrate, ciprofibrate, fenofibrate, and gemfibrozil), the first three can increase serum creatinine in cyclosporine-treated patients. Although all fibrates in combinations with statins have been associated with creatinine kinase elevations with or without overt rhabdomyolysis and myopathy, gemfibrozil may have a greater risk for the development of myopathy compared with bezafibrate or fenofibrate.13
Niacin monotherapy has not been reported to cause myopathy, but its combined use with lovastatin, pravastatin, or simvastatin may be associated with rhabdomyolysis. Bile acid sequestrants must be used with caution because of their potential interference with the absorption of CNIs and mycophenolic acid derivatives. Coadministration of bile acid sequestrants and mycophenolic acid products is not recommended. It should also be noted that studies in the general population suggest that bile acid sequestrants may increase TG levels. A more complete list of drug-drug interaction of statins with other lipid-lowering agents is provided in reference 13.
Statins should be the first-line therapy for the treatment of non–high-density lipoprotein (non-HDL) cholesterol because of their well-established safety and efficacy in preventing CV disease in randomized trials in the general population. Fibrates should be considered in those intolerant to statins despite dose reduction or despite switching to another statin. There are currently no data to suggest that adding fibrates to statin therapy is superior to adding nicotinic acid to statin therapy (or vice versa) in the treatment of post-transplant hypertriglyceridemia. Hence the choice of one agent over the other should be based on adverse effects and potential drug-drug interactions.
For patients with fasting TG levels above 1000 mg/dl (11.3 mmol/l), the Adult Treatment Panel (ATP) III recommends a very-low-fat diet (<15% total calories), medium-chain TGs, and fish oils to replace some long-chain TGs.
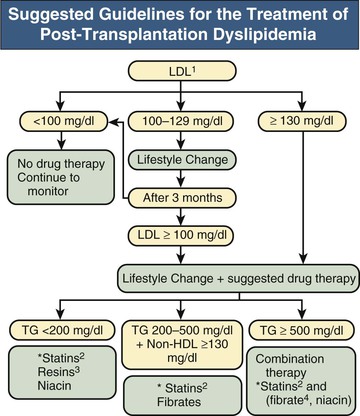
Suggested guidelines for pharmacologic treatment of dyslipidemia are summarized in Figure 106-1.
New-Onset Diabetes After Transplantation
New-onset diabetes mellitus after transplantation (NODAT) occurs in 4% to 25% of kidney transplant recipients. Variations in incidence may result from differences in definition, duration of follow-up, and the presence of both modifiable and nonmodifiable risks factors.16 Major risk factors include African American, Hispanic, and South Asian ethnicity (compared with Caucasians or East Asians), obesity (defined as BMI greater than 30 kg/m2), age older than 40 to 45 years, family history of diabetes among first-degree relatives, impaired glucose tolerance before transplantation or presence of other components of the metabolic syndrome, recipients of deceased donor kidneys, hepatitis C infection, and immunosuppressive therapy including corticosteroids; tacrolimus and to a lesser extent cyclosporine; and the mTOR inhibitors sirolimus and everolimus (Fig. 106-2).16 Neither azathioprine (AZA) nor MMF is diabetogenic.
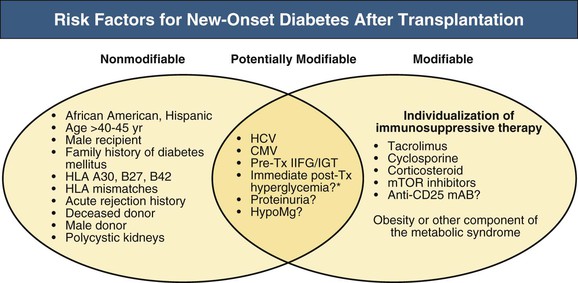
Other potential risk factors for the development of NODAT include the presence of certain human leukocyte antigens (HLAs) (such as A30, B27, and B42), increasing HLA mismatches, acute rejection history, cytomegalovirus (CMV) infection, male recipient, and male donor.16 Polycystic kidney disease has been suggested to confer an increased risk of developing diabetes after kidney transplantation in some studies but not in others. Studies evaluating the association between genetic variations (single nucleotide polymorphism) and NODAT have been inconclusive. The findings of one single-center study suggest that new-onset hyperglycemia in the immediate postoperative period is predictive of future NODAT risk.17
Management of New-Onset Diabetes After Transplantation
In the general population, glycemic management in type 2 diabetes mellitus (T2DM) has become increasingly complex and often controversial because of the uncertain risks and benefits associated with intensive glycemic control and the widening array of pharmacologic agents available.18 Both T2DM and NODAT are characterized by a relative or absolute impairment in insulin secretion, along with varying degrees of peripheral insulin resistance. In essence, NODAT resembles T2DM and it seems plausible to manage NODAT with a stepwise approach similar to that for patients with T2DM.
Patient-Centered Approach
Medical management usually involves a multidisciplinary team approach involving patients (and frequently family members), transplant coordinators, transplant physicians, and diabetic educators. A shared decision-making approach whereby clinicians and patients mutually exchange information and reach a consensus on the therapeutic course of action is helpful.18
Target Hemoglobin A1c Levels
The 2011 American Diabetes Association’s Standards of Medical Care in Diabetes recommend lowering hemoglobin A1c (HbA1c) to below 7.0% in most patients to reduce the incidence of microvascular disease. More stringent control (HbA1c 6.0% to 6.5%) might be considered in a subset of patients with short disease duration, long life expectancy, and no significant CV disease, if this can be achieved without significant hypoglycemia or other treatment-related adverse effects.18 For NODAT, the 2009 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines suggest a target A1C level from 7.0% to 7.5%, not to fall below 6.0%, particularly if hypoglycemic reactions are common. In patients with diabetes, the use of low-dose aspirin (75 mg or 100 mg daily in Europe, 81 mg daily in the United States) for the primary prevention of CV disease should be based on patient preferences and values, balancing the risk of ischemic vascular events with the risk of bleeding.
Therapeutic Interventions
Nonpharmacologic Management
The effect of lifestyle modification to prevent or delay the progression of post-transplantation impaired glucose to overt NODAT has not been studied. A small cross-sectional and observational study showed that higher levels of physical activity were associated with a lower risk of abnormal glucose tolerance and obesity in kidney transplant recipients. In contrast, individuals with limited physical activity consistently showed higher rates of abnormal glucose tolerance and central obesity.19 Because higher pretransplant BMI correlates with insulin resistance after transplantation, obesity treatment seems to be a reasonable target for intervention.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








