Phuong-Thu Pham, Gabriel M. Danovitch, Phuong-Chi T. Pham
Medical Management of the Kidney Transplant Recipient
Infections, Malignant Neoplasms, and Gastrointestinal Disorders
Although patient and graft survival rates in recipients of solid organ transplantation have improved significantly, malignant neoplasms and infectious complications continue to adversely affect post-transplantation morbidity and mortality. Antimicrobial prophylaxis has reduced the incidence of previously common infections such as cytomegalovirus (CMV) or Pneumocystis, but new or resistant microbial pathogens have emerged as a consequence of prophylactic antimicrobials and nosocomial exposures. In addition, donor-derived infections or malignancy can arise as a result of delayed donor seroconversion after a recent acute infection, unidentified pathogens in an organ donor, occult neoplastic disease at the time of organ procurement, or malignant transformation of donor cells. This chapter discusses infections and post-transplantation–related malignant neoplasms in recipients of kidney transplant. Post-transplantation infectious- and drug-related gastrointestinal complications are also discussed.
Infectious Diseases
Infection follows cardiovascular disease as the second most common cause of death with a functioning graft in kidney transplant recipients. Predisposing risk factors include immunosuppression, indwelling Foley or vascular catheters, surgical drains, drug-induced leukopenia, and metabolic derangement. Epidemiologic exposures (travel to regions with endemic pathogens), shifts in nosocomial flora associated with repeated antimicrobial exposures (antimicrobial resistance), and improvement in molecular diagnostic assays have also contributed to the emergence of novel infections and changes in the epidemiology of infections.1
Immunizations Before and After Transplantation
All kidney transplant candidates should receive immunization for hepatitis B, pneumococcus, and other standard immunizations appropriate for age. Up-to-date recommendations for routine adult immunizations are available through the Centers for Disease Control and Prevention website (www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf). Vaccinations should be administered at least 4 to 6 weeks before transplantation to achieve optimal immune response and to minimize the possibility of live vaccine–derived infection in the post-transplantation period. Household members, close contacts, and health care workers should also be fully immunized.
A minimum of 4 weeks should elapse between live virus vaccine administration and transplantation. Live virus or live organism vaccines should be avoided after transplantation. These include measles-mumps-rubella (MMR), live oral polio and smallpox (vaccinia) (which are also contraindicated for household contacts), varicella, yellow fever, adenovirus, live oral typhoid (Ty21a), bacillus Calmette-Guérin (BCG), and intranasal influenza vaccine. In addition, exposure to persons who have chickenpox or herpes zoster should be avoided until the lesions have crusted over and no new lesions are appearing. Immunizations using inactivated or killed microorganisms, components, and recombinant moieties are safe for transplant recipients. These include hepatitis A and hepatitis B, pneumococcal, Haemophilus influenzae type b, inactivated polio, diphtheria-pertussis-tetanus (DPT), and Neisseria meningitidis vaccines.
Infection with influenza A (H1N1) virus has emerged as an important cause of morbidity and mortality in the general and dialysis population. Infected patients on chronic dialysis treatment were found to have a 10-fold higher mortality rate compared with the general population. Recipients of solid organ transplants have similarly been reported to be at risk for more severe disease. Hence, unless contraindicated, influenza A (H1N1) vaccine should be considered in all prospective kidney transplant candidates.
Most centers restart vaccinations 3 to 6 months after transplantation. Vaccination before the first 3 to 6 months after transplantation may result in suboptimal response and protection because of heavy immunosuppression. Currently existing data show no conclusive evidence for a link between vaccination and allograft dysfunction.2 For recommendations for prophylaxis or vaccination for transplant recipients who travel to countries where endemic infections such as malaria are present, please refer to reference 3. Recommended immunizations before and after transplantation are listed in Table 105-1.
Table 105-1
Recommended immunizations before and after transplantation.
All patients who are at least 1 month post-transplant should receive seasonal influenza vaccine.7
| Recommended Immunizations Before and After Transplantation* | ||
| Vaccine | Before Transplantation | After Transplantation |
| Measles-mumps-rubella | X | — |
| Diphtheria-tetanus-pertussis | X | Diphtheria and tetanusa |
| Varicella | X | Contraindicated |
| Poliovirus | X | Inactivated polio vaccineb |
| Haemophilus influenzae type b | X | X |
| Influenza | X | Xc |
| Pneumococcus | X | Xd |
| Hepatitis B | X | Xe |
| Hepatitis A | X | Xf |
| Human papillomavirus (HPV) | Xg | — |
| Neisseria meningitidis | Xh | Xh |
| Zostavax | Xi | — |
a Booster every 10 years.
b For travelers to endemic areas (i.e., some parts of Asia, Africa).
c Annually.
d Every 3-5 years.
e Monitor titers.
f For travelers to endemic areas.
g Nonpregnant female transplant candidates ages 9-26.
h Recommended for military members; travelers to high-risk areas; properdin-deficient or terminal complement component–deficient patients; patients with functional or anatomic asplenia; college freshmen living on campus (American Society of Transplantation Infectious Diseases Guidelines3).
i Consult with transplant infectious disease specialist.
Infectious Causes
Both the type and occurrence of infections in the immunocompromised transplant recipient follow a “timetable pattern” (Table 105-2).1
Table 105-2
Timetable of infections.
Geographically focused infections, such as malaria, leishmaniasis, trypanosomiasis, and strongyloidiasis, will need to be considered in certain patients.
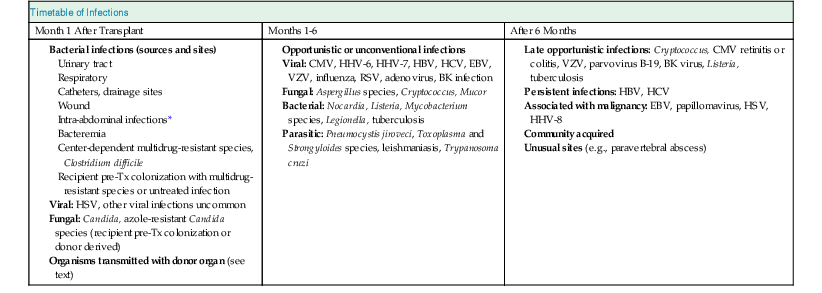
* Sources of infections specific to recipients of renal transplant include perinephric fluid collections (e.g., lymphoceles, wound hematomas, urine leaks), indwelling urinary stents, or anatomic or functional genitourinary tract abnormalities (e.g., ureteral stricture, vesicoureteric reflux, neurogenic bladder). CMV, Cytomegalovirus; EBV, Epstein-Barr virus; HBV, hepatitis B; HCV, hepatitis C; HHV, human herpes virus; HSV, herpes simplex virus; pre-Tx: Pre-transplant; RSV, respiratory syncytial virus; VZV, varicella-zoster virus.
(Modified from reference 59.)
Donor-Derived Infections
Donor-derived infections have remained the focus of increased attention since the 2007 report of transmission of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) from an initially antibody-negative donor to four transplant recipients. Other reported donor-transmitted blood-borne and kidney infections include viral (hepatitis B virus [HBV], CMV, and BK virus [BKV], among others), parasitic (malaria, Babesia, and Balamuthia), bacterial (from undiagnosed bacteremia or renal infections), and fungal (Candida species) infections. In recent years, allograft-transmitted infections including lymphocytic choriomeningitis virus, West Nile virus, amebic meningitis, and rabies have been added to the list of unusual viral infections. In addition, transmission of common organisms including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and azole-resistant Candida species occurs with increased frequency despite routine surgical prophylaxis because of the emergence of antimicrobial resistance in both hospital and community settings. Currently, most organ procurement organizations in the United States perform nucleic acid testing (NAT) in an effort to more precisely identify HIV and hepatitis infections during the window period. High vigilance for donor-derived infections must be maintained.
Month 1 After Transplantation
In the first month after transplantation, donor- and recipient-derived infections with common nosocomial bacterial microorganisms and Candida species predominate. Infections caused by multidrug-resistant bacteria are center specific. Infections caused by multidrug-resistant bacteria including gram-positive bacteria (MRSA, VRE), extended-spectrum β-lactamases (ESBLs), gram-negative bacilli, and multidrug-resistant nonfermentative gram-negative bacilli have emerged as important causes of morbidity and mortality in organ transplantation worldwide. Strict adherence to isolation measures and proper hand washing should not be overlooked.
Most bacterial infections during this period involve wounds, catheters, and drainage sites. Aspiration pneumonia and urinary tract infections (UTIs) are common. The U.S. Renal Data System database revealed a cumulative UTI incidence of 17% during the first 6 months after transplantation and 60% at 3 years for women and 47% for men. Infections specific to kidney transplant recipients include perinephric fluid collections caused by lymphoceles, wound hematomas, or urine leaks; indwelling urinary stents; and UTIs secondary to urinary tract abnormalities, such as ureteral stricture, vesicoureteral reflux, or neurogenic bladder. Most UTIs are caused by gram-negative bacteria (Escherichia coli, Enterobacteriaceae, and Pseudomonas) and gram-positive bacteria (Enterococcus). Preventive measures for UTIs include early urethral catheter removal and antibiotic prophylaxis. Trimethoprim-sulfamethoxazole or ciprofloxacin prophylaxis during the first 3 to 6 months after transplantation may reduce the risk of UTIs threefold4 and essentially eliminates urosepsis unless anatomic or functional derangement of the urinary tract is present. Strict aseptic surgical techniques and perioperative use of first-generation cephalosporins reduce the incidence of wound infections. Although uncommon, donor or recipient pretransplant colonization with multidrug-resistant strains may represent an important risk factor for infection with multidrug resistant bacteria in the post-transplant period. Except for herpes simplex virus (HSV), viral infections are uncommon during this period.
Months 1 to 6
During months 1 to 6, opportunistic infections secondary to immunosuppression are most common. Viral infections such as CMV, HSV, varicella-zoster virus (VZV), Epstein-Barr virus (EBV), HBV, and HCV may occur from exogenous infection or reactivation of latent disease as a result of the immunosuppressed state. Reactivation or de novo HCV infection remains an important cause of morbidity and mortality after transplantation because of the lack of effective antiviral drugs and the risk of precipitating acute allograft rejection associated with interferon therapy. Repeated courses of antibiotics and corticosteroid therapy increase the risk of fungal infections, whereas viral infections not only may result from immunosuppression but may further impair immunity and increase the risk for additional opportunistic infections. Opportunistic infections may occur with Pneumocystis jiroveci (previously called Pneumocystis carinii), Aspergillus species, Listeria monocytogenes, Nocardia species, and Toxoplasma gondii. Trimethoprim-sulfamethoxazole prophylaxis (see Table 105-3 for trimethoprim-sulfamethoxazole allergy) eliminates or reduces the incidence of Pneumocystis, L. monocytogenes meningitis, Nocardia species infection, and T. gondii. Reactivation of latent infection such as Mycobacterium tuberculosis, Trypanosoma cruzi, Leishmania species, Strongyloides stercoralis, Cryptococcus neoformans, Histoplasma capsulatum, and Coccidioides and Paracoccidioides species may be observed. Community-acquired respiratory viruses remain a common hazard in these vulnerable immunocompromised patients during this time period. Clinically, patients may present with pneumonitis, viremia, or tissue-invasive disease such as hepatitis or carditis.1 Over the past two decades, BK polyomavirus–associated clinical syndromes have emerged as important complications in kidney transplant recipients (discussed further under BK infection).
Table 105-3
Suggested prophylactic therapy for recipients of kidney transplants.
CMV, Cytomegalovirus; mTOR, mammalian target of rapamycin; UTI, urinary tract infection.
| Suggested Prophylactic Therapy for Recipients of Kidney Transplants | |
| Regimen | Comments |
| Trimethoprim-sulfamethoxazole (TMP-SMX)* (80/400 mg) one tablet daily × 6-12 mo | Its routine use reduces or eliminates the incidence of Pneumocystis jiroveci, Listeria monocytogenes, Nocardia asteroides, and Toxoplasma gondii. In renal transplant recipients, TMP-SMX × 6 mo reduces the incidence of UTIs threefold. |
| Dapsone† > Atovaquone > monthly aerosolized pentamidine‡ | Replaces TMP-SMX for patients with sulfa allergies. Consider adding fluoroquinolone or another agent for antibacterial activity. |
| Nystatin 100,000 units/ml, 4 ml after meals and before bedtime or Fluconazole§ 200 mg one tablet daily × 2 months | For fungal prophylaxis. Close monitoring of cyclosporine or tacrolimus or mTOR inhibitor levels is required when starting and stopping antifungal agents. |
| Acyclovir, valganciclovir, ganciclovir | For CMV prophylaxis, see Box 105-1. |
* The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines suggest UTI prophylactic therapy for at least 6 months after transplantation. For patients receiving sirolimus immunosuppression, we recommend 1 year of therapy for Pneumocystis pneumonia prophylaxis.
† Check glucose-6-phosphate dehydrogenase deficiency before initiation of therapy.
‡ In order of preference.
§ Fluconazole recommended for recipients of combined kidney-pancreas or combined kidney-liver transplants. Consider reinstituting prophylactic therapy × 3 months after acute rejection episodes requiring intensification of immunosuppression.
After 6 Months
After 6 months, the infection risk is largely a function of chronic maintenance immunosuppression, exposure to T cell–depleting agents, graft function, and epidemiologic exposures. In essence, patients can be arbitrarily divided into three categories in terms of infection risks.
The first category consists of the majority of transplant recipients (70% to 80%), who have satisfactory or good allograft function, relatively low doses of immunosuppressants, and no history of chronic viral infection. The risk of infection is similar to that of the general population, with community-acquired respiratory viruses constituting the major infective agents. Opportunistic infections are unusual unless environmental exposure has occurred.
The second group (approximately 10% of patients) consists of those with chronic viral infection that may include HBV, HCV, CMV, EBV, BKV, or papillomavirus. In the setting of immunosuppression, such viral infections may lead to the development of progressive liver disease or cirrhosis (HBV, HCV), BK nephropathy, post-transplant lymphoproliferative disease (EBV), or squamous cell carcinoma (papillomavirus). The use of alemtuzumab (a humanized monoclonal antibody directed against CD52 found on T and B cells) has been reported to be associated with increased risk of late invasive viral and fungal infections because of its effect on profound and prolonged pan–T cell depletion. New infections occurring in this time period often reflect new exposures (e.g., L. monocytogenes [dietary indiscretions], Lyme disease, and malaria [travelers to endemic areas]).1
The third group (approximately 10% of patients) consists of those who experience multiple episodes of rejection requiring repeated exposure to heavy immunosuppression. These patients are the most likely to develop chronic viral infections and superinfection with opportunistic infections. Causative opportunistic pathogens include Pneumocystis, Listeria, Nocardia, Cryptococcus, and geographically restricted mycoses (coccidioidomycosis, histoplasmosis, blastomycosis, and paracoccidioidomycosis). We advocate lifelong antifungal prophylaxis in high-risk transplant recipients such as those with a history of past infection or those who live in endemic areas. Environmental exposure (primarily avoidance of pigeons and areas of active building construction) should be minimized.
Management and Prophylactic Therapy for Selected Infections
The following section discusses selected important infections and suggested approaches to their management. Suggested prophylactic therapy in kidney transplant recipients is shown in Table 105-3.
Cytomegalovirus Infection
Cytomegalovirus infection may cause primary infection in a seronegative recipient (donor seropositive, recipient seronegative), reactivation of endogenous latent virus (donor seropositive or seronegative, recipient seropositive) or superinfection with a new virus in a seropositive recipient (donor seropositive, recipient seropositive). Primary CMV infection is usually more severe than reactivated infection or superinfection.
Clinical Manifestations
Cytomegalovirus infection may be asymptomatic, presenting as a mononucleosis-like syndrome or influenza-like illness with fever and leukopenia or thrombocytopenia, or a severe systemic disease. Hepatitis, esophagitis, gastroenteritis with colonic ulceration, pneumonia, carditis, and even otitis may occur. In enterically drained pancreas transplantation, CMV has been reported to cause a bleeding ulcer from the duodenal segment. Although chorioretinitis (associated with retinal hemorrhage) is a common manifestation of CMV disease in acquired immunodeficiency syndrome (AIDS) patients, it seldom occurs in solid organ transplant recipients. Nonetheless, any organ system may be affected by CMV. Clinical manifestations usually occur 1 to 4 months after transplantation except for chorioretinitis, which occurs later in the transplant course. Results of quantitative CMV assays of serum in patients with invasive colitis and gastritis or neurologic disease including chorioretinitis are often negative. Diagnosis in such patients may require invasive testing and biopsies.
Immunomodulating Effects of Cytomegalovirus Infection
Cytomegalovirus infection is associated with immune modulation and dysregulation of helper and suppressor T cells. CMV infection may be a risk factor for acute and chronic allograft rejection, secondary infection with opportunistic agents (such as Pneumocystis, Candida, and Aspergillus), and reactivation of human herpesvirus 6 (HHV-6) and HHV-7 and may favor development of post-transplant lymphoproliferative disease. CMV infection is also associated with acceleration of HCV infection and the development of new-onset diabetes after transplantation.5
Risk Factors for Cytomegalovirus Infection
Donor and recipient seropositive status and the use of blood products from CMV-seropositive donors are well-established risk factors for CMV infection. Other factors associated with an increased risk of CMV infection include the use of antilymphocyte antibodies, prolonged or repeated courses of antilymphocyte preparations, comorbid illnesses, concomitant HHV-6 and HHV-7 viral infections, neutropenia, lack of CMV-specific CD4+ and CD8+ T cells, and acute rejection episodes. Mycophenolate mofetil (MMF) has been reported to increase the risk of CMV viremia and disease in some studies, especially in patients receiving more than 3 g/day. Although the cause-effect relationship of allograft rejection and CMV infection remains conjectural, several studies suggest that one may increase the risk of the other, possibly because of the release of inflammatory cytokines. Prevention of CMV infection, for example, results in a lower incidence of graft rejection.6
Prevention
Prophylactic therapy begins in the immediate postoperative period. Preemptive therapy involves treatment of those who are found to seroconvert by quantitative laboratory assays of the blood, such as CMV DNA polymerase chain reaction (PCR) or pp65 antigenemia during surveillance studies. The former assay is highly specific and sensitive for the detection of CMV viremia. The latter is a semiquantitative fluorescent assay in which circulating neutrophils are stained for nonspecific uptake of CMV early antigen (pp65). Screening for CMV is best performed using NAT methods, although CMV antigenemia assays are still being used by some centers. Currently, CMV prophylactic therapy is recommended over initiation of preemptive treatment after detection of CMV viremia or antigenemia.7
Various prophylactic and preemptive protocols have been developed. Oral acyclovir provides effective CMV prophylaxis only in recipients of seronegative donor organs. Oral or intravenous ganciclovir or oral valganciclovir provides superior prophylactic or preemptive therapy against primary CMV infection or CMV reactivation. The 2-year results of the Improved Protection Against Cytomegalovirus in Transplant (IMPACT) study demonstrated that in high-risk CMV D+/R− recipients, once-daily oral valganciclovir prophylaxis for 200 days provided a significant reduction in the incidence of CMV disease compared with the 100-day once-daily prophylaxis. Extended prophylaxis to 200 days did not increase the rate of ganciclovir-resistant CMV disease.8 Furthermore, a cost-effectiveness model also demonstrated that extended prophylaxis using valganciclovir was cost-effective in reducing the incidence of events associated with CMV disease over a 5- and 10-year period.9
Seronegative individuals who receive organs from latently infected seropositive donors are at greatest risk for primary infection and severe CMV disease. Delayed-onset (or late-onset) CMV disease developing soon after completion of antiviral prophylaxis occurs in 15% to 38% of high-risk CMV D+/R− solid organ transplant recipients who received 3-month CMV prophylaxis10 and is associated with poor patient and graft survival. In high-risk kidney transplant patients, the IMPACT study showed that CMV disease developed in 36.8% versus 16.1% in the 100- versus 200-day valganciclovir prophylaxis groups, respectively, at 12 months after transplantation. Other suggested risk factors for delayed-onset CMV disease include allograft rejection, overimmunosuppression, and lack of CMV-specific immunity.11 The Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines suggest oral ganciclovir or valganciclovir in CMV D+/R− or D+/R+ for at least 3 months after transplantation and for 6 weeks after treatment with T cell–depleting antibodies. Suggested CMV prophylaxis protocol is shown in Box 105-1.
Treatment
The 2010 International Consensus guidelines recommend valganciclovir (900 mg orally every 12 hours, adjusted according to glomerular filtration rate [GFR]), or intravenous ganciclovir (5 mg/kg every 12 hours, adjusted according to GFR) for nonsevere CMV disease. In patients with severe or life-threatening disease and in those with impaired absorption or who are intolerant of oral medication, intravenous ganciclovir should be used. Antiviral drug dose reduction because of side effects should be done judiciously to avoid loss of efficacy. Reduction or withholding of mycophenolic acid products, azathioprine (AZA), or trimethoprim-sulfamethoxazole should be considered before valganciclovir or ganciclovir dose reduction. Severe leukopenia (absolute neutrophil count <500 to 1000/mm3) can be treated with granulocyte colony-stimulating factor (G-CSF). Treatment should be continued until clearance of viremia as assessed by PCR or antigenemia, but not shorter than 2 weeks. Reduction of immunosuppression should be considered in severe disease, in slow responders or nonresponders, and in those with high viral loads or leukopenia. The use of CMV immunoglobulin in the treatment of CMV disease may be considered as adjunctive therapy in individuals with hypogammaglobulinemia, in nonresponders to standard therapy, or in those with severe forms of CMV disease such as pneumonitis.12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








