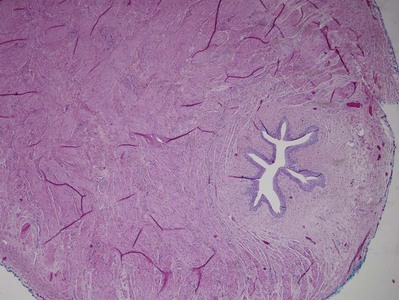
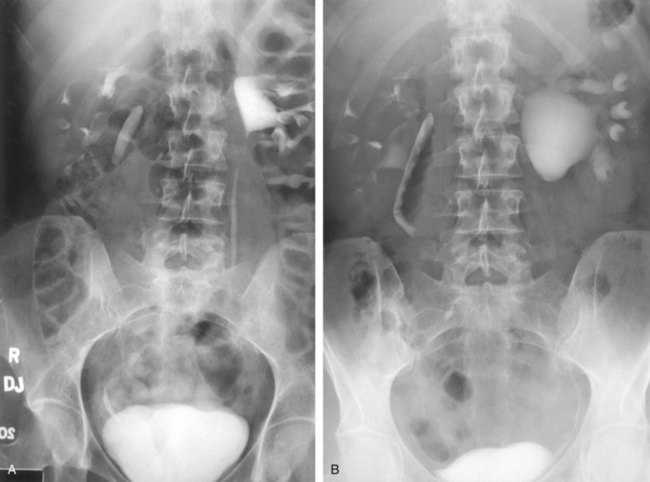
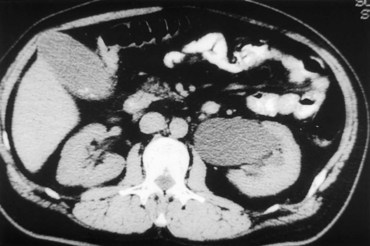
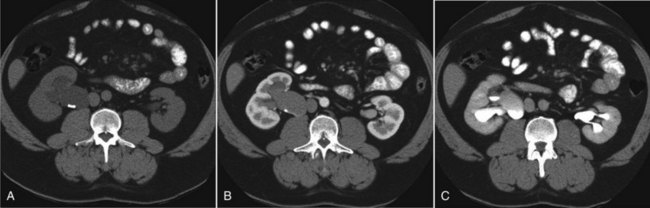
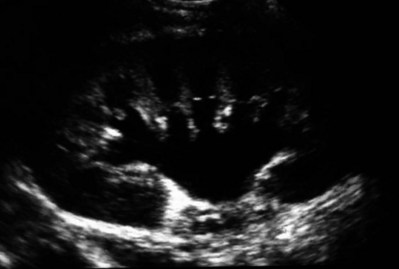
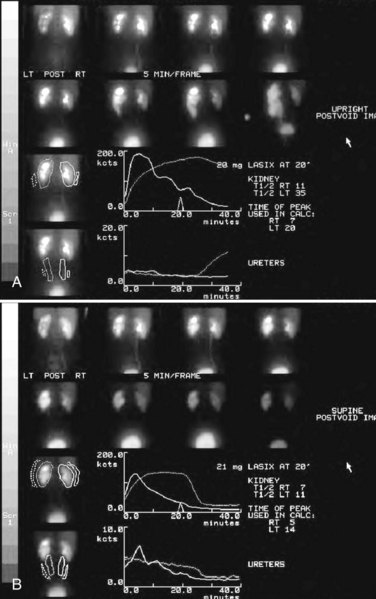
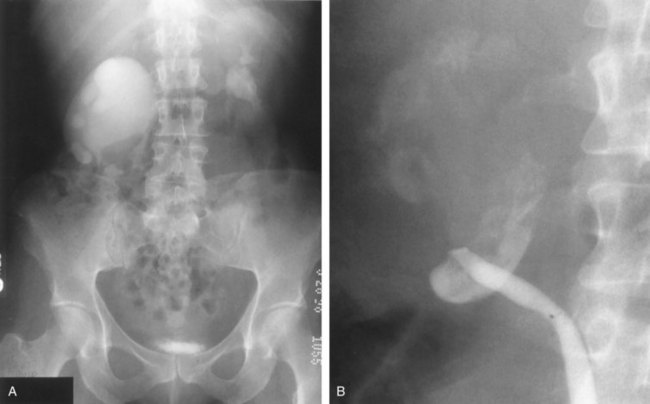
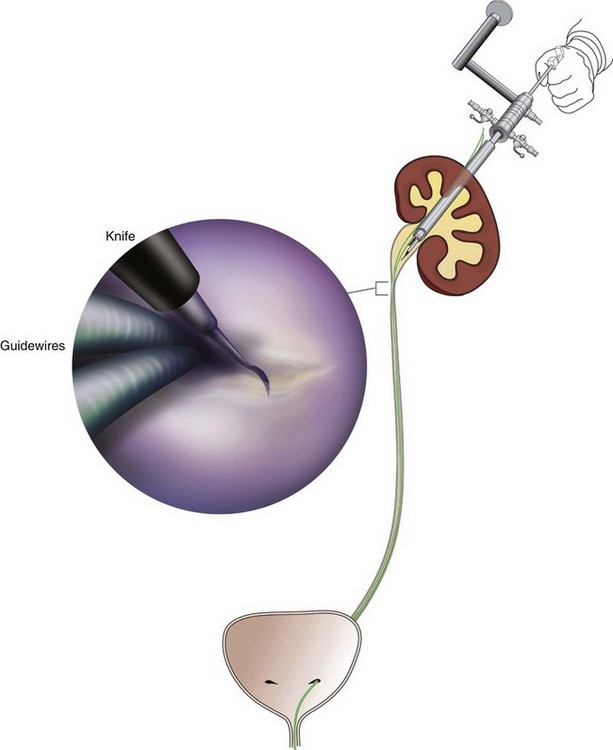
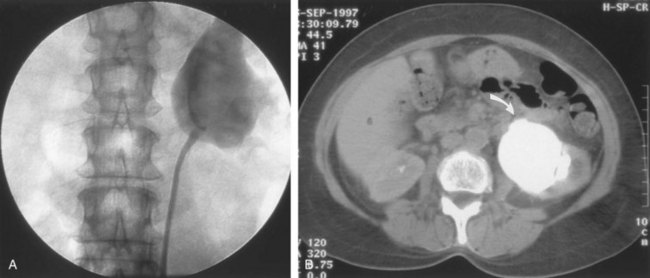
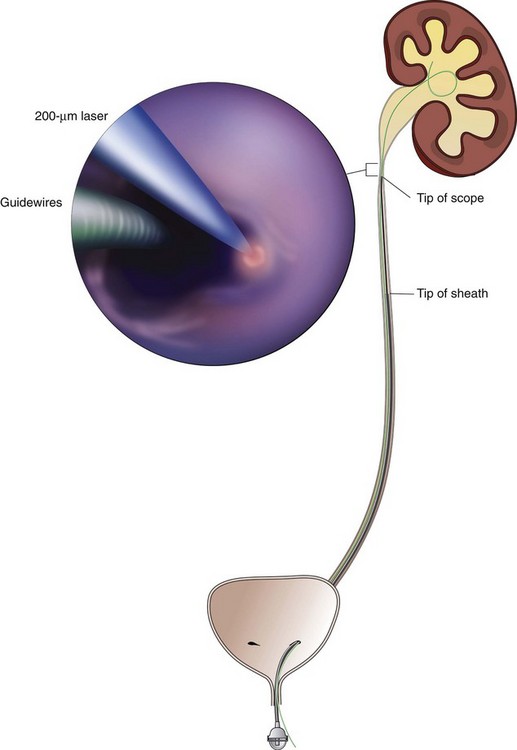
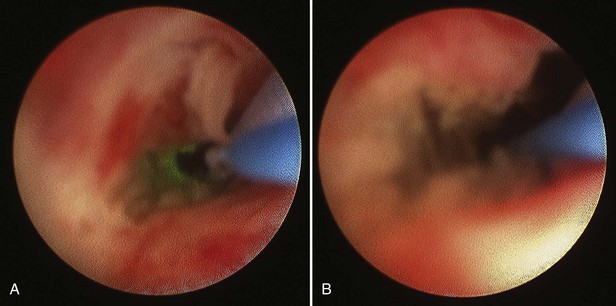
Stephen Y. Nakada, MD, Thomas H.S. Hsu, MD The diagnosis of “ureteropelvic junction obstruction” results in a functionally significant impairment of urinary transport from the renal pelvis to the ureter. Although most cases are congenital, the problem may not become clinically apparent until much later in life (Jacobs et al, 1979). Acquired conditions such as stone disease, postoperative or inflammatory stricture, or urothelial neoplasm may also present clinically with symptoms and signs of obstruction at the level of the ureteropelvic junction (UPJ). Similarly, extrinsic obstruction can occur at this level as well. This section focuses primarily on the diagnosis and treatment of “congenital” UPJ obstruction, although these techniques may be applied to the management of certain acquired conditions, in particular urinary stones. Congenital UPJ obstruction typically results from intrinsic disease. A frequently found defect is the presence of an aperistaltic segment of the ureter, perhaps similar to that found in primary obstructive megaureter. In these cases, histopathologic studies reveal that the spiral musculature normally present has been replaced by abnormal longitudinal muscle bundles or fibrous tissue (Allen, 1970; Foote et al, 1970; Hanna et al, 1976; Gosling and Dixon, 1978) (Fig. 41–1). This results in failure to develop a normal peristaltic wave for propagation of urine from the renal pelvis to the ureter. Recognition that this type of segmental defect is often responsible for UPJ obstruction is of utmost importance clinically because such ureters may appear grossly normal at the time of surgery, and, in fact, may often be calibrated to 14 French or greater. Further investigations in the etiology of UPJ obstruction have shown decreased interstitial cells of Cajal at the UPJ in children (Solari et al, 2003). In addition, the cytokine produced in the urothelium has also been proposed to exacerbate UPJ obstruction (Chiou et al, 2005). Other experimental studies have implicated transforming growth factor-β, epidermal growth factor expression, nitric oxide, and neuropeptide Y in UPJ stenosis (Knerr et al, 2001; Yang et al, 2003). A less frequent intrinsic cause of congenital UPJ obstruction is true ureteral stricture. Such congenital ureteral strictures are most frequently found at the UPJ, although they may be located at sites anywhere along the lumbar ureter. Abnormalities of ureteral musculature have been implicated as electron microscopy has demonstrated excessive collagen deposition at the site of the stricture (Hanna et al, 1976). Intrinsic obstruction at the UPJ may also result from kinks or valves produced by infoldings of the ureteral mucosa and musculature (Maizels and Stephens, 1980). In these cases, the obstruction may actually be at the level of the proximal ureter. This phenomenon appears to result from retention or exaggeration of congenital folds normally found in the ureter of developing fetuses. In some of these cases, the defects are bridged by ureteral adventitia. Grossly, this can manifest as external bands or adhesions that appear to be causing the obstruction. In fact, Johnston and colleagues, in 1977, reported that lysis of external adhesions can at times reestablish flow without pyeloplasty. In the majority of cases, however, these bands or adhesions are likely to be a secondary phenomenon associated with intrinsic obstruction, so operative pyeloplasty would generally be most effective. The presence of these kinks, valves, bands, or adhesions may also produce angulation of the ureter at the lower margin of the renal pelvis in such a manner that, as the pelvis dilates anteriorly and inferiorly, the ureteral insertion is carried further proximally. In these cases, the most dependent portion of the pelvis is inadequately drained and the apparent “high insertion” of the ureteral ostium is actually a secondary phenomenon (Kelalis, 1976). In at least some cases, however, the high insertion itself is likely the primary obstructing lesion because this phenomenon is found more frequently in the presence of renal ectopia or fusion anomalies (Zincke et al, 1974; Das and Amar, 1984). As such, a high insertion can have implications in the subsequent surgical management, particularly endourologic approaches. Controversy persists regarding the potential role of “aberrant” vessels in the etiology of UPJ obstruction. Significant crossing vessels have been noted in up to 63% of cases of UPJ obstruction but as little as 20% of cases of normal kidneys (Quillin et al, 1996; Zeltser et al, 2004; Richstone et al, 2009). Although these lower pole vessels have often been referred to as aberrant, these segmental vessels, which may be branches from the main renal artery or arise directly from the aorta, are usually normal variants (Stephens, 1982). In some patients, these lower pole vessels cross the ureter posteriorly and truly have an aberrant course. Historically, it has been believed that the associated vessel alone does not cause the primary obstruction (Hanna, 1978). In fact, the true etiology is an intrinsic lesion at the UPJ or proximal ureter that causes dilatation and ballooning of the renal pelvis over the polar or aberrant vessel. Recent studies using three-dimensional multidetector row computed tomography (CT) demonstrated that the precise location of crossing vessels did not correspond to the obstructive transition point in patients with UPJ obstruction (Lawler et al, 2005). In contrast, one group found improvement in patients undergoing only ligation of crossing vessels (Keeley, Jr. et al, 1996). Richstone and colleagues reviewed histopathology from 95 patients with UPJ obstruction and found that 43% of 65 patients with a crossing vessel had no intrinsic abnormality (Richstone, 2009). Regardless, the presence of crossing vessels most certainly has a detrimental effect on the success rates of endopyelotomy (Van Cangh et al, 1994; Nakada et al, 1998). UPJ obstruction with concominant anatomic anomalies such as horseshoe kidney and pelvic kidney also present further surgical challenges. Preoperative spiral CT angiographic studies are useful in these patients to identify crossing vessels and to aid in surgical planning (Pozniak et al, 1997). Notably, the current emphasis on laparoscopic and robotic pyeloplasty has quelled the interest in the relevance of preoperative assessment of crossing vessels because this can be addressed at the time of reconstruction. UPJ obstruction may also result from acquired lesions. In children, vesicoureteral reflux can lead to upper tract dilatation with subsequent elongation, tortuosity, and kinking of the ureter. In some cases, these changes may only mimic the radiographic findings of true UPJ obstruction. However, true UPJ obstruction may definitely coexist with vesicoureteral reflux, although it may be difficult to determine whether the anomalies are merely coincident or whether the upper tract ureteral obstruction has resulted from the reflux (Lebowitz and Johan, 1982). Diuretic renography is first line for differentiating between UPJ obstruction and reflux. Other acquired causes of obstruction at the UPJ include benign lesions such as fibroepithelial polyps (Berger et al, 1982; Macksood et al, 1985), urothelial malignancy, stone disease, and postinflammatory or postoperative scarring or ischemia. For these acquired diseases, the techniques discussed in this section may be useful adjuncts for management of the obstruction as long as the primary problem is also addressed where appropriate. For instance, fibroepithelial polyps can be managed using retrograde ureteroscopy and holmium laser excision (Lam et al, 2003a). UPJ obstruction, although most often a congenital problem, can present clinically at any time of life. Historically, the most common presentation in neonates and infants was the finding of a palpable flank mass. However, the current widespread use of maternal, prenatal ultrasonography has led to a dramatic increase in the number of asymptomatic newborns being diagnosed with hydronephrosis, many of whom are subsequently found to have UPJ obstruction (Bernstein et al, 1988; Wolpert et al, 1989). A fraction of cases may also be found during evaluation of azotemia, which may result from bilateral obstruction in a functionally or anatomically solitary kidney. UPJ obstruction may also be incidentally found during studies performed to evaluate unrelated anomalies such as congenital heart disease (Roth and Gonzales, 1983). In older children or adults, intermittent abdominal or flank pain, at times associated with nausea or vomiting, is a frequent presenting symptom. Hematuria, either spontaneous or associated with otherwise relatively minor trauma, may also be an initial symptom. Laboratory findings of microhematuria, pyuria, or frank urinary tract infection might also bring an otherwise asymptomatic patient to the urologist. Rarely, hypertension may be a presenting finding (Riehle and Vaughan, 1981). Radiographic studies should be performed with a goal of determining both the anatomic site and the functional significance of an apparent obstruction. Although excretory urography remains a reasonable option for radiographic diagnosis, this study is less commonly used today. Classically, excretory urographic findings include delay in function associated with a dilated pelvicalyceal system. If the ureter is visualized, it should be of normal caliber. In some patients, symptoms may be intermittent and urography between painful episodes may be normal. In such cases the study should be repeated during an acute episode when the patient is symptomatic (Nesbit, 1956). Provocative testing with diuretic urography may allow accurate diagnosis in select cases. The patient should be well hydrated and the study then performed after injecting furosemide, 0.3 to 0.5 mg/kg (Malek, 1983) (Fig. 41–2). CT scan is frequently obtained for any patient presenting with acute flank pain (Fielding et al, 1997; Dalrymple et al, 1998; Vieweg et al, 1998) (Fig. 41–3). Moreover, CT scans provide detailed anatomic and functional information to aid in diagnosis of UPJ obstruction (Fig. 41–4A-C). Both ultrasonography and CT scanning also have a role in differentiating acquired causes of obstruction such as radiolucent calculi or urothelial tumors. In neonates and infants, the diagnosis of UPJ obstruction has generally been suggested either by routine performance of maternal ultrasonography or by the finding of a flank mass. In either setting, renal ultrasonography is usually the first radiographic study performed. Ideally, ultrasonography should be able to visualize dilatation of the collecting system to help differentiate UPJ obstruction from multicystic kidney and determine the level of obstruction. UPJ obstruction and multicystic kidneys are distinguishable in the majority of cases by ultrasound alone. With UPJ obstruction, the pelvis is visualized as a large, medial sonolucent area surrounded by smaller, rounded sonolucent structures representing dilated calyces. At times, dilated calyces will be seen connecting to the pelvis via dilated infundibula (Fig. 41–5). Occasionally, a solid-appearing renal cortex can be seen surrounding the sonolucent areas or separating the dilated calyces. In contrast, the cysts of multicystic kidneys are visualized as various-sized sonolucent areas in random distribution. Although the cysts may be connected, this is rarely visualized sonographically. Furthermore, little solid tissue is seen and that which is present has a random distribution among the cysts. Rarely, a large, centrally located cyst may cause confusion in the diagnosis (King et al, 1984a). In this setting, nuclear renography should be performed. Specifically, a technetium Tc99m-diethylenetriamenepentacetic acid (99mTc-DTPA) scan allows differentiation of these two entities. Multicystic kidneys rarely reveal concentration of this isotope. When uptake is seen, the areas of functioning tissue are initially discrete and are usually medial to the bulk of the mass, which itself remains a “cold” area. In contrast, neonatal kidneys with UPJ obstruction generally exhibit good concentration of the isotope. Furthermore, even with severe obstruction in which only a cortical rim remains, uptake of the isotope will be seen peripherally in the cortex, again helping to differentiate this from multicystic kidney (King et al, 1984a). Diuretic renography is effective in predicting recoverability of function in cases in which intravenous urography has revealed nonvisualization. Diuretic renography allows quantification of the degree of obstruction and can help differentiate the level of obstruction. Today, 99mTc-MAG3 is the preferred isotope because of favorable imaging and dosimetry considerations over 99mTc-DTPA or radioiodinated hippuran (Roarke, 1998). Diuretic renography remains a commonly used study for diagnosing both UPJ and ureteral obstruction because it provides quantitative data regarding differential renal function and obstruction, even in hydronephrotic renal units. Diuretic renography is noninvasive and readily available in most medical centers. Ideally diuretic renography can be used to follow patients for functional loss, most effectively when a standard protocol is used. The diuretic is given 20 minutes into the study to allow time for filling of the collecting system. One study found diuretic renography to be useful in children to rule out concomitant UPJ obstruction with associated high-grade reflux (Stauss et al, 2003). There is evidence that the diuretic renography using MAG-3 is a most accurate study for patients with UPJ obstruction following therapeutic intervention (Niemczyk et al, 1999) (Fig. 41–6). The diagnosis of UPJ obstruction can generally be made with a high degree of certainty on the basis of the clinical presentation and the results of any one or more of the imaging studies already cited. It is preferable to have a combination of anatomic and functional studies, such as retrograde pyelogram and diuretic renography, in order to best plan therapy. Retrograde pyelography thus retains a role for confirmation of the diagnosis and for demonstration of the exact site and nature of obstruction before repair. In most cases, this study is performed at the time of the planned operative intervention in order to avoid the risk of introducing infection in the face of obstruction. However, retrograde pyelography is indicated emergently whenever the UPJ obstruction requires acute decompression, such as in the setting of infection or compromised renal function. In cases in which cystoscopic retrograde manipulation has been unsuccessful or may be hazardous, particularly in neonates or infants, placement of a percutaneous nephrostomy is preferred. This allows the performance of antegrade studies that will help define the nature and exact anatomic site of obstruction. It also allows decompression of the system in cases of associated infection or compromised renal function and allows assessment of recoverability of renal function after decompression. When there remains some doubt as to the clinical significance of a dilated collecting system, placement of a percutaneous nephrostomy tube allows access for dynamic pressure perfusion studies. First described by Whitaker in 1973, the renal pelvis is continuously perfused at 10 mL/min with normal saline solution or dilute radiographic contrast solution under fluoroscopic control. Renal pelvic pressure is monitored during the infusion, and the pressure gradient across the UPJ is determined. During the infusion, the bladder is continuously drained with an indwelling catheter to prevent transmission of intravesical pressures. Renal pelvic pressure ranging up to 12 to 15 cm H2O during this infusion suggests a nonobstructed system. In contrast, pressures in excess of 15 to 22 cm H2O are highly suggestive of a functional obstruction. Pressures between these extremes may be nondiagnostic (O’Reilly, 1986). Although pressure perfusion studies can often provide valuable information regarding the functional significance of an apparent obstruction, these studies can at times be inaccurate. This inaccuracy may be a result of variations in renal pelvic anatomy and compliance (Koff et al, 1986) or positional variations (Ellis et al, 1995). As such, the urologist must be a diagnostician and collate the clinical presentation and results of all diagnostic studies performed in order to identify the best clinical intervention. Contemporary indications for intervention for UPJ obstruction include the presence of symptoms associated with the obstruction, impairment of overall renal function or progressive impairment of ipsilateral function, development of stones or infection, or, rarely, causal hypertension. The primary goal of intervention is relief of symptoms and preservation or improvement of renal function. Traditionally, such intervention should be a reconstructive procedure aimed at restoring nonobstructed urinary flow. This is especially true for neonates, infants, or children in whom early repair is desirable because these patients will have the best chance for improvement in renal function after relief of obstruction (Bejjani and Belman, 1982; Roth and Gonzales, 1983; Wolpert et al, 1989). However, timing of the repair in neonates remains controversial (DiSandro and Kogan, 1998; Koff, 1998, 2000; Hanna, 2000; Shokeir and Nijman, 2000), mostly because of difficulty in defining those kidneys truly at risk for functional obstruction. In a prospective study of 104 neonates with primary unilateral hydronephrosis suspected of being caused by UPJ obstruction, after a mean follow-up of 21 months, only 7 (7%) required pyeloplasty for functional obstruction, defined as a progression of hydronephrosis or a 10% reduction in differential glomerular filtration rate on serial ultrasonography and diuretic renography (Koff and Campbell, 1994). All treated patients had a return of renal function to predetermination levels, supporting selective nonoperative management of neonatal hydronephrosis. UPJ obstruction may not become apparent until middle age or later (Jacobs et al, 1979). Occasionally, if the patient is asymptomatic and the physiologic significance of the obstruction seems indeterminate, careful observation with serial follow-up studies may be appropriate, typically using diuretic renography. However, the majority of affected patients may ultimately benefit from reconstructive intervention (Jacobs et al, 1979; Clark and Malek, 1987; O’Reilly, 1989). When intervention is indicated, the procedure of choice has historically been dismembered pyeloplasty. However, less invasive endourologic approaches have a role as an alternative in many centers (Brannen et al, 1988; Motola et al, 1993a; Kletscher et al, 1995; Cohen et al, 1996; Nadler et al, 1996; Thomas et al, 1996; Tawfiek et al, 1998; Lechevallier et al, 1999; Gerber and Kim, 2000; Nakada, 2000; Conlin, 2002). Most recently, laparoscopic and robotic pyeloplasty has gained acceptance as primary therapy at centers with appropriate skills and technology (DiMarco et al, 2006; Rassweiler et al, 2007). Although success rates with most endourologic techniques have not proven to be comparable with those of open, laparoscopic, or robotic pyeloplasty, it has been suggested that the success rates may be significantly improved with careful patient selection. In an important prospective study, Van Cangh and colleagues (1994) achieved an overall success rate for endopyelotomy of 73%. However, these investigators found the presence of crossing vessels to be a major determinant of outcome (42% success rate in the setting of a crossing vessel vs. 86% success without a crossing vessel). Furthermore, when endopyelotomy was applied to patients with “a high degree of obstruction,” the success rate was only 60% compared with an 81% success rate for those patients with “low-grade” obstruction. When patients with both a crossing vessel and a high degree of obstruction were excluded from analysis, the success rate improved to 95%, which is comparable with that of open pyeloplasty. However, other studies have suggested a less important role for these factors in regard to their impact on a successful outcome (Gupta et al, 1997; Danuser et al, 1998; Nakada et al, 1998). Although the indications for intervention for UPJ obstruction are similar regardless of technique, it is critical to discuss the risks and benefits of all available options with patients. As such, each patient should be advised individually on the basis of all the anatomic and functional information available preoperatively. In this setting, many patients will opt for a minimally invasive approach, even with the understanding that success rates may be lower, or secondary intervention may become necessary. As a result of studies linking crossing vessels to hindered endourologic successes, there is increased interest in intraoperative management of the UPJ and crossing vessel by either an open or laparoscopic approach (Conlin, 2002). Therefore for “secondary” UPJ obstruction, it remains reasonable to recommend an open or laparoscopic approach to any patient who has failed primary endourologic management and an endourologic approach to those who have failed open or laparoscopic repair. The results of endourologic management in this setting remain generally excellent (Jabbour et al, 1998; Canes et al, 2008). Operative intervention for UPJ obstruction has historically provided a widely patent, dependently positioned, well-funneled UPJ. In addition, the option to reduce the size of the renal pelvis is readily available with this approach. Although pyeloplasty has stood the test of time with a published success rate of 95%, several less invasive alternatives to standard operative reconstruction exist (Clark et al, 1987). The advantages of endourologic approaches include reduced hospital stays and postoperative recovery. However, the success rate does not approach that of open, laparoscopic, or robotic pyeloplasty. Furthermore, whereas open, laparoscopic, or robotic pyeloplasty can be applied to almost any anatomic variation of UPJ obstruction, consideration of any of the less invasive alternatives require that the surgeon take into account the degree of hydronephrosis, ipsilateral renal function, concomitant calculi, and possibly the presence of crossing vessels. Of note, Albani and colleagues (2004) reported contemporary long-term results with various endopyelotomy approaches to have a success rate of 67%, with the majority of failures in the first 32 months. More recently, DiMarco and colleagues (2006) reported long-term follow-up of more than 400 patients undergoing either percutaneous antegrade endopyelotomy or pyeloplasty. The 3-, 5-, and 10-year success rates were superior for pyeloplasty, 85% versus 63%, 80% versus 55%, and 75% versus 41%. Moreover, Rassweiler and colleagues (2007) compared retrograde laser endopyelotomy with laparoscopic retroperitoneal pyeloplasty in 256 patients in a 10-year single surgeon experience and found success rates were 73% for laser endopyelotomy compared with 94% for pyeloplasty. Endourologic management of UPJ obstruction was introduced by Ramsay and colleagues in 1984 as a “percutaneous pyelolysis” and then popularized in the United States by Badlani and colleagues (1986), who coined the term “endopyelotomy.” Although various nuances in the technique have been described (Korth et al, 1988; Van Cangh et al, 1989; Ono et al, 1992), the basic concept of the endopyelotomy is a full-thickness lateral incision through the obstructing proximal ureter, from the ureteral lumen out to the peripelvic and periureteral fat. A stent is placed across the incision and left to heal, in keeping with the original work of Davis in 1943, who performed an “intubated ureterotomy” to repair UPJ obstruction. Subsequently, alternative techniques using a retrograde approach to the UPJ were developed. The retrograde approach most used today is the ureteroscopic approach, typically using the holmium laser to incise the UPJ under direct visual control. Alternatively, a cautery wire balloon endopyelotomy, which incises the UPJ under fluoroscopic control, or percutaneous endopyeloplasty may be used (Gill et al, 2002). Recently, Vaarala and colleagues reported a small series of 64 patients who underwent either antegrade or retrograde cold knife or cautery wire balloon endopyelotomy. In this study, success rates ranged from 79% to 83%, without statistically significant differences among the three treatments (Vaarala et al, 2008). Of note, transplantation complications are particularly suited to endoscopic management, either antegrade or retrograde (Schumacher et al, 2006; Gdor et al, 2008a). As far as efficacy is concerned, there is little evidence for significant differences between endopyelotomy techniques. The differences lie in technical considerations and complications. The indications to intervene for any patient with UPJ obstruction include the presence of symptoms, progressive or overall impairment of renal function, development of upper tract stones or infection, or, rarely, causal hypertension. Historically, a percutaneous approach for definitive management of UPJ obstruction was offered only to those patients undergoing percutaneous removal of associated stones or to those who had previously failed open pyeloplasty. However, encouraging results ultimately led many centers to offer percutaneous endopyelotomy as primary therapy for almost any patient with UPJ obstruction. Even with the acceptance of laparoscopic pyeloplasty, percutaneous endopyelotomy is also appropriate for those patients with UPJ obstruction and concomitant pyelocalyceal stones, which can then be managed simultaneously. Contraindications to a percutaneous endopyelotomy are similar to the contraindications to any endourologic approach and include a long segment (>2 cm) of obstruction, active infection, or untreated coagulopathy. Whereas the impact of crossing vessels is controversial, the mere presence of crossing vessels is not a contraindication to an endopyelotomy (Motola et al, 1993b; Nakada et al, 1998; Lam et al, 2003b). However, significant entanglement of the UPJ by crossing vessels can occasionally be identified and this may render any endourologic approach unsuccessful. When such entanglement is suggested by intravenous or retrograde pyelography (Fig. 41–7), it can be reliably verified using three-dimensional helical CT (Kumon et al, 1997). In the original descriptions of the technique both from the Institute of Urology in London (Ramsay et al, 1984) and from Long Island Jewish Hospital in New York (Badlani et al, 1986), the endopyelotomy was performed using a cold knife technique under direct vision. With one or two wires in place across the UPJ, a direct vision “endopyelotome” is used. This hook-shaped cold knife may be used to completely incise the UPJ in a full-thickness manner, from the ureteral lumen to periureteral and peripelvic fat (Fig. 41–8). Rigorous anatomic studies have shown the incision should generally be made laterally because this is the location devoid of crossing vessels (Sampaio, 1998). However, in cases of high insertion, the incision should instead “marsupialize” the proximal ureter into the renal pelvis, such that an anterior or posterior incision may be required (Fig. 41–9). When such incisions are done under direct vision, any crossing vessel can be directly visualized and avoided. In addition to the endopyelotome, the holmium laser or the cutting balloon catheter may also be used to perform an antegrade endopyelotomy. Once the incision is complete, stenting is accomplished. There remains no consensus as to the optimal stent size or duration for endopyelotomy. A No. 14/7-Fr endopyelotomy stent may be used, passed in an antegrade fashion with the larger diameter end of the stent positioned across the UPJ. In some cases, especially when the patient has not been prestented, passage of this large-caliber stent may be difficult. In those instances, a No. 10/7-Fr endopyelotomy stent or even a standard No. 8-Fr internal stent may be used without compromising the ultimate outcome. Once proper positioning of the stent is determined fluoroscopically, any remaining safety wires are withdrawn. One group showed no difference between larger and standard stents in a porcine study of endopyelotomies (Moon et al, 1995). Alternatively, Danuser and colleagues (2001) demonstrated improved success rates using a modified 27-Fr stent following percutaneous endopyelotomy at nearly 2 years follow-up. Avoidance of strenuous activity for 8 to 10 days after the procedure is recommended. The ideal stent size, duration of stent placement, and radiographic follow-up after endopyelotomy remains unclear (Canes et al, 2008). One study did report a benefit to larger stents in patients undergoing antegrade endopyelotomy (71% vs. 93%); however, a large-bore (27-Fr) catheter was used for the initial 3 weeks postoperatively (Danuser et al, 2001). On the other hand, Kletscher and colleagues (1995) reported no benefit to larger stents as did Hwang and colleagues (1996). Wolf and colleagues (1997) reported improved success using larger stents (12 Fr) in endoureterotomy patients in a retrospective review. Regarding stent duration, less is known. The original report and recommendation of 6 weeks by Davis (1943) is still often used, although Mandhani and colleagues (2003) identified no difference in results when comparing 57 patients stented for 2 weeks compared with 4 weeks. Although the need for prophylactic antibiotics while the stent is indwelling is not literature based, many use a daily suppressive dose. Once the stent is removed, the patient returns 1 month later for clinical follow-up and radiographic evaluation. This generally includes a history, physical, urinalysis, and diuretic renography. If the patient remains asymptomatic and the diuretic renography reveals normal drainage (normal The immediate and long-term results of percutaneous endopyelotomy are well established. Although percutaneous endopyelotomy compares favorably with open operative pyeloplasty in terms of postoperative pain, length of hospital stay, and return to prehospitalization activities (Brooks et al, 1995; Karlin et al, 1988), retrograde endopyelotomy and laparoscopy also offer favorable convalescence. Gerber and Lyon, in 1994, reviewed the outcome of percutaneous endopyelotomy in 672 patients reported from 12 centers and found a success rate ranging from 57% to 100% (mean, 73.5%) at follow-up ranging from 2 to 96 months. Currently, success rates approaching 85% to 90% are being reported at experienced centers, with little difference in outcome noted in those patients undergoing the procedure for primary versus secondary UPJ obstruction (Motola et al, 1993a; Kletscher et al, 1995; Shalhav et al, 1998). Of note, Knudsen and colleagues (2004) reported long-term results in 80 patients using the cold knife and holmium laser for antegrade endopyelotomy, with 55-month follow-up. This series had a success rate of 67%, slightly lower than otherwise reported. Interestingly, DiMarco and colleagues (2006) reported on 182 antegrade endopyelotomies with a recurrence-free survival at a single center over 10 years as low as 41%. Of note, Schumacher and colleagues (2006) reported on three successful antegrade endopyelotomies in transplanted kidneys in 2006. When percutaneous endopyelotomy does fail, several options exist including a retrograde endopyelotomy, repeat percutaneous endopyelotomy, or laparoscopic, robotic, or open operative intervention. There remains a role for spiral CT angiography in failed endopyelotomy, to rule out a crossing vessel. If a significant vessel is found, repeat endopyelotomy is generally not recommended (Nakada, 2000). Alternatively, operative intervention is generally offered to any patient who has failed an endourologic approach. On the basis of available data, the results of laparoscopic pyeloplasty will not be compromised (Motola et al, 1993b; Gupta et al, 1997; Conlin et al 2002). The complications associated with percutaneous endopyelotomy are analogous to those associated with percutaneous nephrolithotomy (Badlani et al, 1988; Weiss et al, 1988; Cassis et al, 1991; Malden et al, 1992; Bellman, 1996), and hemorrhage is a risk of any percutaneous upper tract procedure including endopyelotomy. However, because in patients with UPJ obstruction the renal parenchyma is generally thinner than that associated with a normal kidney, and because the collecting system is dilated, this risk may be different than that in the general population of stone patients undergoing percutaneous manipulation. Acute management in this setting is generally conservative to start: bed rest, hydration, and transfusion if necessary. The nephrostomy tube should not be irrigated acutely. Rather, it is preferable to allow the pyelocalyceal system to tamponade the bleeding. When continued bleeding does not respond to these conservative measures, the next step is selective angiographic embolization. Generally, the urologist should have a low threshold to proceeding to angiography in order to minimize the need for transfusion and potential exploration. Successful angiographic embolization often obviates the need for operative “exploration” that can lead to nephrectomy. Percutaneous endopyeloplasty is a hybrid technique described as an endoscopic Heineke Mikuliz repair performed through a percutaneous tract. In other words, endopyeloplasty combines percutaneous endopyelotomy and an endoscopic Fenger plasty. Initial and short-term reports by Gill and colleagues have been published demonstrating both feasibility and safety, and success rates have been similar to percutaneous endopyelotomy in limited comparative series (Gill et al, 2002; Canes et al, 2008). Latest reports include 55 patients with short-term follow-up with more than 90% success by these same authors (Stein et al, 2007). Early reports identified endopyeloplasty to be more effective in primary rather than secondary UPJ obstruction, most likely because tissue scarring may inhibit the endoscopic reconstruction. Percutaneous endopyelotomy is particularly favorable when the UPJ obstruction is associated with upper tract stone disease because the stones can be managed concomitantly. In such cases, percutaneous access is again established with a wire across the UPJ. The stone should be removed before the endopyelotomy so that stone fragments do not migrate into the peripyeloureteral tissue, as can happen if the endopyelotomy is performed first. Otherwise, localized obstruction may result from fibrosis or granuloma formation (Giddens et al, 2000; Streem, 2000). The urologist must take care to assure that the UPJ obstruction is not a result of edema from the concominant stone disease, in particular with stone disease in the renal pelvis. In this circumstance, initial management of the stone percutaneously and subsequent radiographic assessment of the UPJ once the stone has been removed are most prudent. In addition, if a nephrostomy tube is retained, a Whitaker test is straightforward and definitive to assess for persistent obstruction. Conversely, UPJ obstruction and solitary lower pole calculi do not represent a dilemma regarding UPJ edema, and combined percutaneous management remains most efficient. Alternatively, laparoscopic pyeloplasty and concominant stone removal is also effective for these patients. A ureteroscopic approach to endopyelotomy was first suggested in 1985 when Bagley and colleagues reported a combined percutaneous and flexible ureteroscopic procedure approach for management of an “obliterated” UPJ. Subsequently, Inglis and Tolley (1986) reported a ureteroscopic “pyelolysis” for UPJ obstruction. Shortly thereafter, Clayman and colleagues (1990) reported an initial experience in a small number of patients performing ureteroscopic endopyelotomy with a No. 3- or 5-Fr cutting electrode passed under direct vision using large, rigid or flexible ureteroscopes. In that series, however, a No. 8-Fr nephrostomy tube was placed at the outset of the procedure and left indwelling for at least 48 hours. As such, that series still represented a “combined” endourologic approach to endopyelotomy. Stents were routinely left in place for 6 to 8 weeks, after which diagnostic studies were performed. With a mean follow-up approaching 1 year, a success rate of 81% was achieved in 16 patients. However, two patients developed distal ureteral strictures, probably resulting from the larger-diameter rigid instrumentation. Thomas and colleagues (1996) subsequently reported their experience with ureteroscopic endopyelotomy. Again, only relatively larger-diameter ureteroscopic instrumentation was available such that preoperative stent placement was routine, and some male patients required perineal urethrostomy. The endopyelotomy incision itself was performed with either cold knife or electrocautery attachments to the ureteroscope. The authors achieved a success rate of approximately 90%, although nephrectomy was ultimately performed in two patients, one of which was done urgently for bleeding. Cold knife ureteroscopic endopyelotomies are still being reported (e.g., Butani and Eschghi reported on a single surgeon experience with 135 cases from 1998-2004). Although three rigid ureteroscope and preprocedure stents were necessary, this group identified 96% success rates in primary procedures with an average 5-year follow-up (Butani and Eschghi, 2008). Notably, the complication rate was only 2.7%. Advances in instrumentation and technique now allow a ureteroscopic approach to be performed reliably at a single setting (Conlin and Bagley, 1998), and this is now considered the standard. The main advantage of a ureteroscopic approach is that it allows direct visualization of the UPJ and assurance of a properly situated, full-thickness endopyelotomy incision without the need for percutaneous access. Another advantage of the ureteroscopic approach is a decrease in cost compared with the use of the cautery wire balloon, assuming ureteroscopic equipment and electroincision or holmium laser is already available. Moreover, the risks and morbidity of percutaneous access are avoided with the ureteroscopic procedure. Gettman and colleagues found that the retrograde ureteroscopic endopyelotomy was more cost effective than hot wire cutting balloon endopyelotomy, antegrade endopyelotomy, and pyeloplasty for treating UPJ obstruction when taking into account treatment failures (Gettman et al, 2003). The indications and contraindications to a ureteroscopic endopyelotomy include functionally significant obstruction, as defined earlier. Contraindications include relatively long areas of obstruction and upper tract stones, which are best managed simultaneously with alternative approaches, usually percutaneously or laparoscopically. Another consideration is that in patients with significant hydronephrosis, the evidence indicates an antegrade endopyelotomy may be more efficacious (Lam et al, 2003b). General anesthesia is used in order to minimize patient movement during ureteroscopy and the subsequent incision of the UPJ. In preparation for the endopyelotomy, a retrograde pyelogram is performed under fluoroscopic control at the outset of the procedure. A hydrophilic guidewire is passed cystoscopically under fluoroscopic control and coiled in the pyelocalyceal system. The cystoscope is then withdrawn and exchanged for the semirigid ureteroscope. The ureteroscope is passed alongside the guidewire to the level of the UPJ. If the distal ureter is too narrow to allow easy passage of the ureteroscope, the intramural ureter can be dilated using a 5-mm balloon or a No. 9- or 10-Fr “introducing” catheter. If the ureter is still too narrow at any point to easily accommodate the ureteroscope, then an internal stent is placed and the procedure postponed for 5 to 10 days to allow “passive” ureteral dilatation. Alternatively, an actively deflecting flexible ureteroscope may be used, and in most cases a ureteral access sheath is quite useful. The sheath allows for rapid transfer of the ureteroscope for assessment of the UPJ. Once the flexible ureteroscope is passed to the UPJ, a 200-µm holmium fiber is placed through the working channel and the UPJ incised in the appropriate location, as suggested by the radiographic studies (Figs. 41-10 and 41-11). The incision is carried caudally into normal ureteral tissue, until the UPJ is widely patent. Injection of contrast material through the ureteroscope can demonstrate extravasation and confirm an adequate depth of incision, although this is generally not necessary because the entire procedure has been performed under direct vision. Balloon dilation up to 24-Fr can also be performed to complete the incision. If any small bleeding points are visualized ureteroscopically, they can be treated by defocusing the holmium laser. Similarly, the balloon can be reinflated to allow tamponade for 10 minutes to see if the bleeding will subside. The ureteroscope is then withdrawn from the ureter while the safety wire is left in place in the renal pelvis for subsequent passage of a stent. Experimental studies have shown that 36-Fr balloon dilation alone can create linear incisions in the UPJ (Pearle et al, 1994). Although retrograde balloon dilation alone has been reported for treatment of UPJ obstruction, long-term follow-up studies have shown a diminishing success rate over time, as low as 42% (McClinton et al, 1993; Webber et al, 1997). Biyani and colleagues (1997) described their initial experience with a ureteroscopic approach using holmium laser energy. With a mean follow-up of slightly more than 12 months, they achieved a success rate of 87.5% in a small group of patients. One patient developed a urinoma, which was managed conservatively. In 1998, Renner and colleagues reported a larger series of patients undergoing ureteroscopic laser endopyelotomy. Using a semirigid ureteroscope, the UPJ was incised at a posterolateral location unless vessels were visualized in that area, in which case a contralateral incision was made. Tawfiek and colleagues (1998) reported the Jefferson Medical College experience with ureteroscopic endopyelotomy. These investigators combined endoluminal ultrasound with their ureteroscopic approach in order to definitively identify crossing vessels or a ureteropelvic septum, which is present in patients with high-inserting ureters. The authors believed this helped them definitively site their endopyelotomy incision. Different modalities were used for the endopyelotomy itself including electrocautery and holmium laser. An 87.5% success rate was achieved in 32 patients. There were no significant bleeding complications, and all patients were discharged within 24 hours of the procedure. Gerber and colleagues and Matin and colleagues reported experiences with ureteroscopic holmium laser endopyelotomy, demonstrating success rates of 70% to 80% with follow-up out to 5 years (Gerber et al, 2000; Matin et al, 2003). More recently, Yanke reported on 128 retrograde ureteroscopic endopyelotomies with a 60% success rate at 20 months while Rassweiler and colleagues reported 73% success in 113 patients at 63 months (Rassweiler et al, 2007; Yanke et al, 2008). Improved results were reported by Conlin and associates (91% success rates) with retrograde endopyelotomy in patients when culling patients with crossing vessels greater than 4 mm using preoperative ultrasonography (Conlin, 2002). Giddens and colleagues also published excellent results when culling patients with anterior and posterior crossing vessels from retrograde endopyelotomy using endoluminal ultrasound (Giddens et al, 2000). To date, the use of endoluminal ultrasound to identify crossing vessels has been controversial, and although it may play a role in preoperative decision making, similar data can be obtained using the less invasive spiral CT angiography. Regardless, the best endopyelotomy success rates still lag behind those of open or laparoscopic pyeloplasty. Complications of this approach have diminished in frequency and severity with the refinement of ureteroscopic instrumentation and the introduction of small-caliber holmium laser fibers. Postprocedural ureteral strictures are rare in contemporary series, and angiographic embolization and nephrectomy are rare using the retrograde approach. Most complications are minor and relate primarily to urinary leak, stent migration, and infection (Gerber and Kim, 2000; Tawfiek et al, 1998). Recently Castle and colleagues reported on ureteroarterial fistula 2 weeks following retrograde laser endopyelotomy, which could be fulgurated ureteroscopically (Castle et al, 2009). Use of a cautery wire balloon for management of UPJ obstruction was first reported in a clinical series by Chandhoke and colleagues in 1993. Use of this device gained initial acceptance by many clinicians because standard cystoscopic techniques and real-time fluoroscopy are all that is necessary for its use. Because the procedure is guided fluoroscopically, such vessels may increase the risk of hemorrhage after activation of the cautery wire balloon. Some authors recommended preoperative imaging for such vessels with relatively noninvasive techniques such as CT or three-dimensional CT angiography (Fig. 41–12) (Streem and Geisinger, 1995; Quillin et al, 1996; Nakada et al, 1998; Herts et al, 1999; Nakada, 2000). Nadler and colleagues (1996) reported on 28 patients 2 or more years after cautery wire balloon endopyelotomy. With a mean follow-up of 32.5 months, subjective improvement was noted in 61% of patients, and 81% had a patent UPJ on the basis of diuretic renography or Whitaker testing. More recent studies have demonstrated lower success rates than these initial series (32% to 63%) and perhaps that high-grade hydronephrosis has a negative impact on success (Albani et al, 2004; Sofras et al, 2004). El-Nahas and colleagues reported a small prospective randomized trial comparing retrograde ureteroscopic endopyelotomy to the hot-wire balloon endopyelotomy in 40 patients. Although not statistically significant, they found superior success rates (85% compared with 65%) and lower complication rates with the ureteroscopic endopyelotomy (El-Nahas et al, 2006). Ponsky and Streem reported on 64 patients undergoing either ureteroscopic endopyelotomy or hot wire balloon endopyelotomy and found equivalent success rates with both procedures yet higher major complication rates in the cautery wire balloon endopyelotomy, specifically transfusion and selective embolization (Ponsky and Streem, 2006). The major complication associated with cautery wire balloon incision is hemorrhage. Although injury to crossing vessels has been reported using the cutting balloon catheter, strict adherence to lateral incision principles minimizes this risk (Sampaio et al, 1993
Ureteropelvic Junction Obstruction
Pathogenesis
Patient Presentation and Diagnostic Studies
Indications and Options for Intervention
Options for Intervention
Endourologic Management
Percutaneous Antegrade Endopyelotomy
Indications and Contraindications
Technique
Postoperative Care
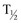 ), reevaluation is performed at 6 months and then at 12-month intervals. Most literature indicates that the majority of endopyelotomy failures occur within the first year of the procedure; however, longer-term studies demonstrate failures well beyond that timeframe (Nadler et al, 1996; Albani et al, 2004; DiMarco et al, 2006; Doo et al, 2007). For most adults, 2- and 3-year follow-up is justified because studies indicate even at 36 months some late failures are identified, but relatively few are identified at 60 months (Doo et al, 2007).
), reevaluation is performed at 6 months and then at 12-month intervals. Most literature indicates that the majority of endopyelotomy failures occur within the first year of the procedure; however, longer-term studies demonstrate failures well beyond that timeframe (Nadler et al, 1996; Albani et al, 2004; DiMarco et al, 2006; Doo et al, 2007). For most adults, 2- and 3-year follow-up is justified because studies indicate even at 36 months some late failures are identified, but relatively few are identified at 60 months (Doo et al, 2007).
Results
Complications
Percutaneous Endopyeloplasty
Simultaneous Percutaneous Endopyelotomy and Nephrolithotomy
Retrograde Ureteroscopic Endopyelotomy
Indications and Contraindications
Technique
Results
Complications
Retrograde Cautery Wire Balloon Endopyelotomy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree