Monitoring frequency guidelines
CKD stage
GFR range (mL/min/1.73 m2)
PTH (pg/mL)
Alkaline phosphatase (U/L)
Calcium and phosphorus (mg/dL)
CKD1 T
>90
Once in the first 3 months and then every 12 months
–
Weekly in the first 3 months till levels are normal and then every 6–12 months
CKD2 T
60–90
–
CKD3 T
30–59
Every 12 months
CKD4 T
15–29
Once in the first 3 months and then every 6–12 months
Every 12 months
Weekly in the first 3 months till levels are normal and then every 3–6 month
CKD5 T
<15
Once in the first 3 months and then every 3–6 months
Every 12 months
Weekly in the first 3 months till levels are normal and then every 1–3 months
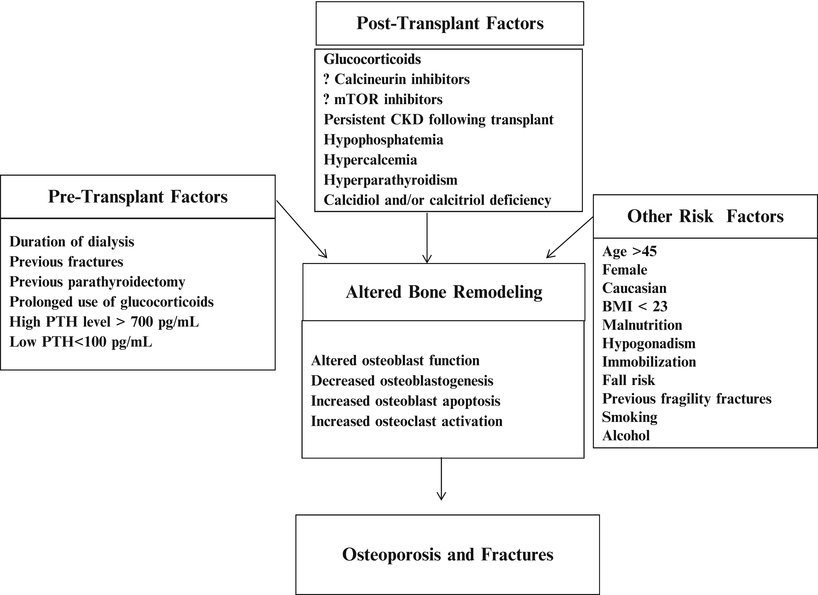
Fig. 24.1
The risk factors and pathogenesis of post-transplant bone loss and post-transplant fracture: the pathogenesis of post-transplant bone disease is multifactorial including but not limited to pre-transplant-related factors, transplant-related factors, and factors that increase the risk of osteoporosis and fractures. All these factors result in uncoupling bone formation and bone resorption with higher rate of the latter and subsequently increase bone loss and bone fractures. CKD chronic kidney disease, PTH parathyroid hormone, BMI body mass index [9–29]
Bone Biomarkers: Biomarkers of bone formation (bone-specific alkaline phosphatase, osteocalcin, procollagen C-terminal propeptide (PICP), and procollagen N-terminal PINP) and bone resorption (urinary collagen breakdown products, TRAP5b) have been used to predict osteoporosis and fractures in the normal population. However, these biomarkers correlate poorly with bone loss as measured by densitometry and bone histology in the transplant patient and have not been shown to predict fracture risk. Until further information is available, there is probably no utility to measure these markers in the post-transplant patient.
Radiographic Evaluation
Densitometry
Bone densitometry, most commonly performed by dual-energy X-ray absorptiometry (DXA), is a readily available, noninvasive, rapid, cost-effective, and relatively precise method of screening BMD with low radiation exposure. However, the usefulness of DXA scans in patients with CKD and post-transplantation is limited because they do not reflect bone quality, turnover, or architecture [66]. The scans do not distinguish the underlying cause of bone loss. Low BMD measurements in post-kidney transplant recipients may be a consequence of osteoporosis and/or osteodystrophic bone disease [67]. The World Health Organization (WHO) [68] criteria defining fracture risk for osteopenia and osteoporosis are based on studies performed in normal postmenopausal Caucasian populations, thus limiting their applicability to kidney transplant recipients. Furthermore, there is no proven predictive relationship between BMD and fracture risk in kidney transplant recipients; in fact at least one study demonstrated that BMD did not distinguish those who went on to experience a fracture from those who did not [55]. BMD measurements in kidney transplant patients should not be looked at in isolation but should be analyzed and considered in the setting of a clinical assessment with biochemical data and possibly bone histology. However despite all of these limitations, BMDs may have a role in the transplant population. Sequential measurements of BMD may be helpful to guide antiresorptive therapy. KDIGO guidelines recommend measuring BMD at the time of transplantation or within the first 3 months after transplantation [23, 56, 69] when estimated GFR is >30 mL/min/m2, particularly in patients with risk factors for rapid bone loss. However, KDIGO does not recommend DXA scans in patients with eGFR < 30 mL/min/1.73 m2 as they are of no proven benefit in predicting fracture risk or the type of post-transplant bone disease [56]. DXA scans can be repeated at 12-month intervals if BMD decreases by greater than 5 % or half of a standard deviation; otherwise, DXA scan can be obtained every 2 years [70] to monitor response to therapy or to help decide whether therapy should be continued.
Conventional Radiographic Studies
Conventional radiography is helpful in identifying fractures, however is of limited value in distinguishing the different bone lesions of renal osteodystrophy. Lateral spine radiographs may be obtained to screen for asymptomatic spinal fragility fractures.
High-Resolution Quantitative Computed Tomography
High-resolution quantitative computed tomography (HR-QCT) is an accurate, noninvasive method of measuring volumetric BMD. It provides high-resolution three-dimensional images with information about volumetric BMD and microarchitecture with separation between cortical and trabecular bone [71]. Changes in volumetric vertebral BMD were found to correlate with changes detected in trabecular bone volume by bone biopsy [71]. Additionally, the trabecular microarchitecture in the radius and tibia using HR-QCT appears to better predict fracture risk in postmenopausal women [72] and is better than DXA in determining risk of fracture in patients with CKD [73, 74]. Unfortunately, this technology is not yet widely available, is costly, and has the disadvantage of high radiation exposure. The utility of this technique in the evaluation of post-transplant bone disease needs to be further evaluated before used outside of the research environment.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a noninvasive screening and diagnostic modality for the early detection of osteonecrosis [75]. The newest generation of high-resolution MRI scanners can also be used to evaluate bone microarchitecture, resulting in images that may represent a “virtual bone biopsy” [76]. A pilot study of micro-MRI in 17 young adults on maintenance hemodialysis compared with healthy controls demonstrated cortical thinning and disruption of the trabecular architecture in the former [77]. Two other preliminary studies have demonstrated that transplant recipients had significantly lower MRI measurements of bone volume and microarchitecture compared with controls; however, spine QCT, trabecular BMD, and DXA hip and spine BMD did not differ between transplant recipients and controls [78, 79] Micro-MRI appears to be a promising tool; however, more data are required to determine its utility in assessing bone disease in the post-transplant patient.
General Measures
The goals of therapy for post-transplant bone disease include increasing bone mass to avoid fractures, normalizing bone turnover, minimizing the risk of osteonecrosis, and optimizing calciotropic and phosphotropic mineral metabolism. Prevention and treatment of post-transplant bone loss and fracture reduction should begin in the pre-transplant period by following preventive measures that are of proven benefit in both the CKD and general population [80], such as limiting the use of steroids, engaging in 30 min of daily vigorous physical exercise, avoiding nicotine and alcohol consumption, and fall risk reduction. Management of the disorders associated with CKD-MBD including hyperparathyroidism, calcium and phosphorus derangements, and treatment of vitamin D insufficiency and deficiency is appropriate.
In the post-transplant period, calcium and vitamin D supplementation can be considered in all kidney transplant recipients who do not have hypercalcemia; however, the effects of vitamin D on BMD in post-transplant recipients are still controversial [51, 81]. Although short-term use of gonadal hormone therapy in solid organ transplant recipients with hypogonadism may slow bone loss, this therapy may increase the risk of coronary artery disease, cancer, and thrombotic events and should not be used routinely [82]. Therapy of post-transplant hypophosphatemia should be considered only in symptomatic patients or patients with serum levels below 1–1.5 mg/dL, given the risk of hypocalcemia, nephrocalcinosis [83], and persistent hyperparathyroidism [84]. When used phosphate supplementation should be closely monitored.
Glucocorticoids Minimizing Protocols
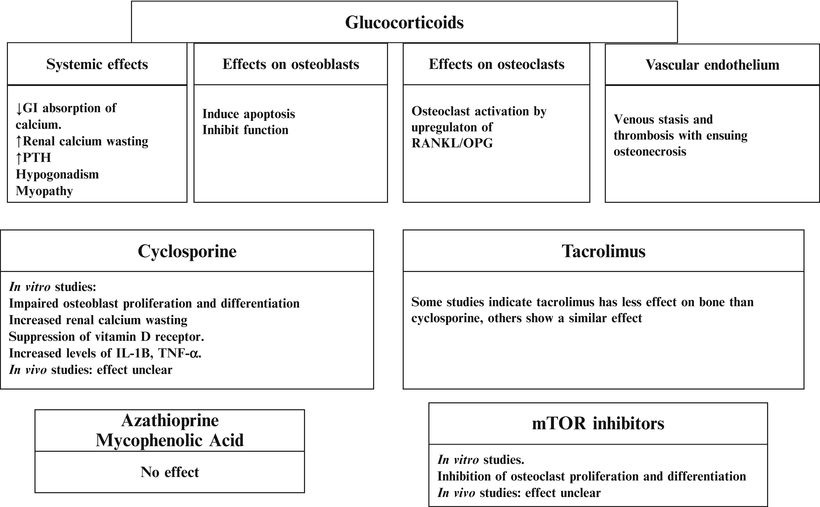
The use of high steroid doses in the early post-transplant period has been associated with a substantial and accelerated bone loss in the first 6–12 months post-kidney transplant [1–3, 69, 85]. In the short term, glucocorticoids profoundly inhibit bone formation and increase bone resorption. With long-term exposure, steroids are associated with low bone turnover and increased apoptosis of both osteoblasts and osteoclasts [86] (Fig. 24.2). The increasing use of steroid-free immunosuppression [87] with steroid withdrawal initiated at a variety of post-transplant time points including 4 days, 3 or 12 months has been associated with preserved and improved BMD at the femoral neck and lumbar spine [88–90]. In addition, minimizing steroid therapy has resulted in less osteonecrosis [61]. To date, there are no data linking the improvement in BMD observed in transplant patients withdrawn from steroids with a reduction in fracture risk. Some observational studies suggest that steroid-free regimens [87] and early steroid withdrawal (discharge from the hospital off steroids after renal transplantation) [88] are associated with decreased incident fractures after the first-year post-transplantation. This finding is not uniform as at least one observational study demonstrated that steroid withdrawal 2–6 months after solid organ transplantation did not result in decreased fractures [91]. Prospective randomized controlled trials are needed to evaluate the role of steroid free regimens or the timing of steroid free regimens withdrawal on fracture risk reduction.
Antiresorptive Therapy
Antiresorptive therapy may be indicated for patients with osteopenia or osteoporosis, who have no evidence of low bone turnover or refractory hyperparathyroidism, and preserved renal function (eGFR > 35 mL/min) in the first post-transplant year [56]. Consideration of antiresorptive therapy use is warranted if the patient is on a steroid-based immunosuppression protocol and/or has a history of fragility fractures [23].
Bisphosphonates
Bisphosphonates are synthetic analogues of pyrophosphates that bind to hydroxyapatite in the bone and directly inhibit osteoclastic bone resorption [92–94]. Bisphosphonates also reduce osteoclastic activity by decreasing osteoclast progenitor development and recruitment and by promoting osteoclast apoptosis [92–94]. As well bisphosphonates prevent osteocyte and osteoblast apoptosis [95]. Data from clinical trials show that both oral and intravenous bisphosphonates prevent bone loss in the lumbar spine and femoral neck in the first post-transplant year [33–43] and beyond [55]. There is a lack of evidence, however, that bisphosphonate use has any beneficial effect on bone strength or fracture prevention in the kidney transplant population. This lack of supporting data may be because of the underpowered nature of studies undertaken to examine this issue. When dosed appropriately in properly selected patients and used for limited periods of time, bisphosphonate therapy is well tolerated, safe, and effective for the prevention and management of post-transplant bone loss. Intravenous zoledronic acid or ibandronate can be an alternative to oral bisphosphonates in patients who cannot tolerate the oral formulation or have difficulty with the dosing requirements. Bisphosphonates should be used with caution in patients with reduced kidney function and their use should be avoided in patients with eGFR of less than 30–35 mL/min, given the prolonged duration of action and accumulation in bone resulting in the possibility of inducing or perpetuating adynamic bone disease [40, 96]. To reiterate, though bisphosphonates have been demonstrated to attenuate bone loss following transplantation, they have not been shown to reduce fractures. In fact, one study demonstrated increased fractures in patients receiving bisphosphonate therapy [55]. Bisphosphonate therapy is generally contraindicated in patients with evidence of low bone turnover [40], and obtaining a bone biopsy before institution of bisphosphonate therapy is the most accurate means of characterizing bone turnover and avoiding its use in adynamic bone states.
A Cochrane review of 24 trials, including 1,209 kidney transplant recipients, evaluating the risks and benefits of bisphosphonates, vitamin D analogues, and calcitonin versus no treatment found that all of the interventions provided clinically significant prevention of bone loss in both the femoral neck and lumbar spine, with the exception of calcitonin [38] which did not improve femoral neck BMD [33]. Bisphosphonates were superior to calcitriol in preserving BMD. No single therapy was found to prevent fractures; however, lack of demonstration does not necessarily mean they do not reduce fractures, because as noted earlier, none of these trials were powered to detect a reduction in fracture risk. However, combining the effect of all therapies versus no intervention, there was a 49 % reduction in the relative fracture risk (confidence interval 0.27–0.99). In another meta-analysis by Stein et al., 11 trials evaluated fracture incidence in 780 solid organ transplant recipients (4 of the 11 trials and 42 % of the patients had kidney transplants) that received either bisphosphonates or vitamin D receptor agonists (VDRAs) within the first-year post-transplant [97]. There were 134 reported fractures in these studies. The use of bisphosphonates or VDRAs was associated with a reduced number of subjects with any fracture and vertebral fractures specifically. The discordance in findings between the Cochrane review and the meta-analysis by Stein et al. may be attributable to discrepancies in the methodology of the analyses, differences between the study populations, the timing of initiation of therapy and most importantly that not all studies included reported on fractures. Specifically in the Palmer et al. review, only 11/24 studies (34/514 patients) had reported fracture data [33], while in the Stein et al. review, 134 incident fractures were reported in the 659 subjects who had follow-up information [97]. These data suggest that preventive therapy including bisphosphonates or VDRAs is likely better than no therapy or calcium alone in preventing post-transplant bone loss and perhaps even fractures. In summary, bisphosphonates may have a role in select patient populations: specifically those at risk for fracture but without evidence for low turnover bone disease. The appropriate duration of therapy is not known; however, a maximum of 12–18 months of therapy immediately post-transplantation may be sufficient. Prospective appropriately controlled studies are required to determine the optimal role of bisphosphonate therapy.
Vitamin D Receptor Agonists
VDRAs can preserve and improve BMD by several mechanisms. VDRAs promote differentiation of osteoblast precursors into mature cells, significantly suppress osteoclastic bone activity, and maintain trabecular bone volume and wall thickness [98, 99]. In addition VDRAs can reverse glucocorticoid-induced decreases in intestinal calcium absorption and mitigate against secondary hyperparathyroidism [99].
Some studies demonstrate that oral calcitriol lowers PTH in patients with persistent post-transplant hyperparathyroidism [100, 101]. Several placebo controlled clinical trials demonstrate that active vitamin D preserves and improves BMD at both the lumbar spine and femoral neck in renal allograft recipients [45–49]. None of these studies show that VDRAs have any deleterious effects on renal allograft function. Clinically important outcomes such as mortality, hospitalizations, or fractures were not evaluated in these studies. Although, bisphosphonates have been shown to be superior to VDRAs in preserving and improving BMD in clinical trials [33, 102, 103], there is emerging evidence that suggests the use of either bisphosphonates or VDRAs in solid organ transplant recipients prevents fractures in the first post-transplant year [97]. Table 24.2 summarizes the indications, risks, and contraindications of VDRA therapy.
Table 24.2
Therapies for post-transplant bone disease
Therapy | Indications | Effect on BMD | Effect on bone fracture | Side effects | Contraindications | Monitoring of therapy |
|---|---|---|---|---|---|---|
Bisphosphonates | 1. Improvement of BMD in kidney transplant recipients with eGFR > 30 mL/min/1.73 m2 who have osteopenia or osteoporosis in the first 12 months post-transplant, after excluding low turnover bone disease | Improve femoral neck and lumbar spine BMDa | May decrease fracture riskb | Risk of adynamic bone disease and subsequent fractures, especially if used in patients with low eGFR | 1. Low turnover bone disease | Bone biomarkers may be helpful |
2. Prevention of bone loss in kidney transplant recipients who have normal BMD but are at high risk of fractures after excluding low bone turnover
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|