Ahmad Abou-Saleh, Stephen C. Bain, David J.A. Goldsmith
Management of the Diabetic Patient with Chronic Kidney Disease
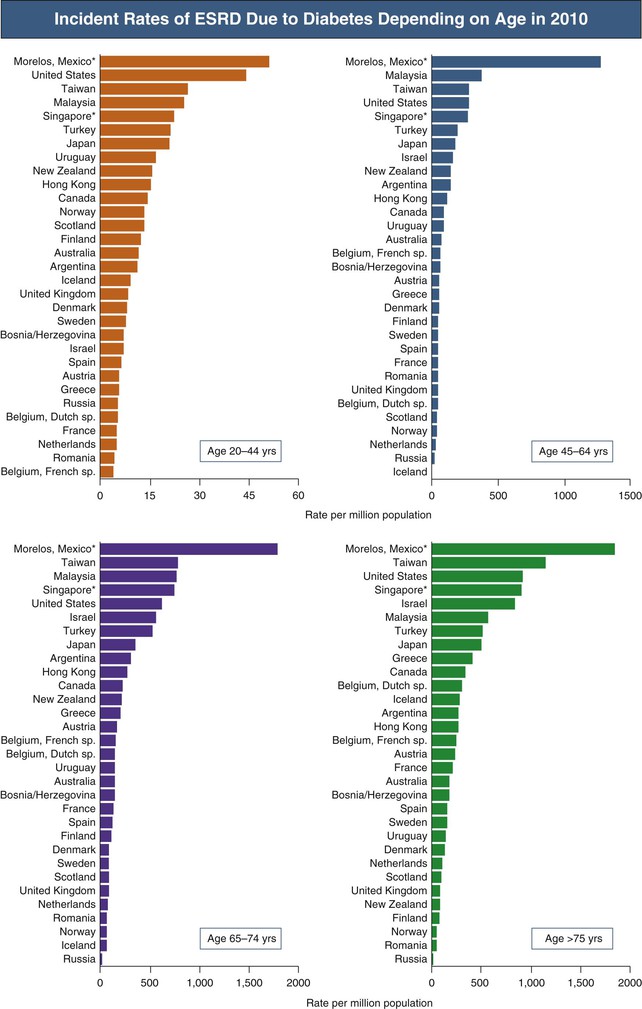
Diabetes mellitus is the leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) (Fig. 32-1). Diabetes is also the most common primary diagnosis in patients receiving renal replacement therapy (RRT).1 After the diagnosis of diabetic nephropathy (DN) has been established, the focus of care must be as follows:
• Aggressive management of diabetes and other risk factors, which are essentially the same for both type 1 and type 2 diabetes mellitus, to prevent or delay the progression of DN (Table 32-1).
Table 32-1
Standard care and target values proposed for patients with diabetes mellitus who have chronic kidney disease (CKD).
ACE, Angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ESA, erythropoiesis-stimulating agent; LDL, low-density lipoprotein; ?, benefit unknown; +/++/+++, moderately/very/highly indicated.
| Standard Care and Target Values Proposed for Diabetic CKD Patients | ||
| Parameter | CKD 3 and 4 | CKD 5 and Dialysis |
| Metabolic Control | ||
| Glycosylated hemoglobin | >6.5-7.5% | >7.0-8.0% |
| Preferred agents | Meglitinides, sulfonylureas, insulin | Insulin |
| Blood Pressure | ||
| Systolic/diastolic BP | 130/80 mm Hg | |
| Preferred agents | ACE/ARBs | ? |
| Lipid Treatment | ||
| LDL cholesterol | <100 mg/dl | ? |
| Preferred agents | Statins | ? |
| Anemia Treatment | ||
| Hemoglobin level | 11.0-12.0 g/dl | 11.0-12.0 g/dl (avoid >13) |
| Preferred agents | Iron/ESA | Iron/ESA |
| Vitamin D Supplements* | ||
| Vitamin D3/1,25-OH D3 | 1,25-OH D3/vitamin D3 | |
| Supportive Treatment | ||
| Smoking cessation | ++ | NP |
| Hypoglycemia awareness | ++ | +++ |
| Low-dose aspirin | ++ | + |
| Exercise (daily/weekly) | + | + |
| Foot care | +++ | +++ |
| Prevention of falls | + | +++ |
* In case of vitamin D supplements, the therapeutic approach should be reversed from native vitamin D first in CKD 3/4 to vitamin D analogues first in CKD5.
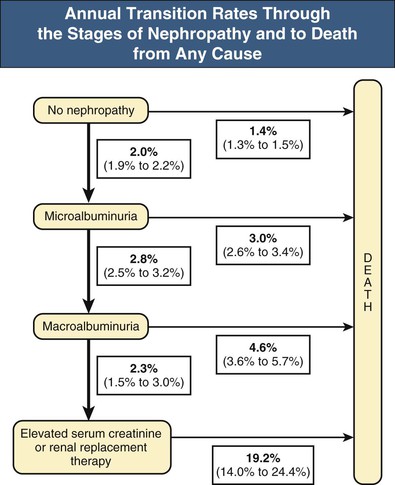
This diabetic CKD patient population will benefit from a multidisciplinary approach, first and foremost with the involvement of nephrologists and diabetologists, but also with input from other specialists, such as cardiologists, renal or diabetes nurse specialists, podiatrists, and dieticians. Early referral to nephrology is also important to rule out other causes of renal disease, especially in patients without evidence of microvascular disease elsewhere (e.g. diabetic retinopathy) or with rapid progression of disease despite well-controlled diabetes. The primary goal in these patients should be stabilization of underlying kidney disease and preventing further progression. Regular follow-up is recommended, from a minimum of yearly appointments, to quarterly appointments in those with progressive CKD. As diabetic CKD progresses through various stages, from normoalbuminuria to microalbuminuria, frank proteinuria, and eventual deterioration in plasma creatinine levels, the risk of cardiovascular death increases (Fig. 32-2).
Hyperglycemia
Assessment
The reliability of glycosylated hemoglobin (HbA1c) as a marker of glycemic control diminishes as CKD progresses. Anemia, iron deficiency, hemolysis, and reduced red blood cell (RBC) life span cause reductions in the measured HbA1c regardless of actual plasma glucose levels, thus underestimating the degree of hyperglycemia. The use of erythropoiesis-stimulating agents can also interfere, generating younger RBCs with reduced exposure to plasma glucose. Therefore, using the “usual” measures for glucose averages and variability is challenging. For example, analysis of data from the Diabetes Control and Complications Trial (DCCT) showed a correlation coefficient of 0.82 with mean plasma glucose in type 1 diabetic patients,2 whereas the correlation coefficients from various studies assessing how closely HbA1c levels relate to mean plasma glucose in patients with CKD have been found to be generally much lower than this (correlation coefficients ~0.5).
Despite these limitations, HbA1c remains a marker of mortality in patients with diabetes-induced CKD, even up to ESRD. The recent Dialysis Outcomes and Practice Patterns Study (DOPPS) data, looking at more than 9000 dialyzed type 1 and type 2 diabetic patients in 12 countries, found that HbA1c strongly predicted mortality as levels increased beyond the range of 7% to 7.9%, which was associated with the lowest mortality rates.3 Lower HbA1c levels were associated with increased mortality as well, particularly in patients with indicators of poor nutritional status.
Other markers of hyperglycemia include glycated albumin and fructosamine, although these are less readily available than HbA1c and are affected by conditions that alter protein metabolism. Newer glycated albumin assays free from the interference of other glycated molecules suggest that it may correlate more accurately than HbA1c with mean plasma glucose, especially in the patient with advanced CKD.4
Treatment
Progressive CKD leads to changes in insulin and carbohydrate metabolism. As glomerular filtration rate (GFR) continues to decline, especially below 60 ml/min/1.73 m2, regular review of the patient’s oral antidiabetic agents or insulin doses is essential because these may need to be reduced or even stopped altogether, a result of accumulation of the drugs and their metabolites, which can have various adverse effects.
Biguanides
The only drug in the biguanide class in contemporary use is metformin, which works as an insulin sensitizer. The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) state that the use of metformin is safe down to an estimated GFR (eGFR) of 30 ml/min/1.73 m2, with dose reductions advised with eGFR less than 45 ml/min/1.73 m2, based on the guidelines issued by the UK National Institute for Clinical Excellence (NICE).5,6 These guidelines help to minimize the risk of lactic acidosis in a population with multiple comorbidities and an increased risk of cardiovascular disease (CVD) and hospitalization. Despite this, lactic acidosis remains extremely rare in clinical trials and cohort studies on metformin therapy, with a Cochrane meta-analysis showing only 4.3 cases per 100,000 patient-years (which was actually less than the nonmetformin group).7
Sulfonylureas
A class of insulin secretagogues, the older generation of sulfonylureas (tolbutamide, glibenclamide) are long acting and almost exclusively excreted by the kidneys and thus are best avoided in CKD patients. Newer agents are of shorter duration and primarily metabolized by the liver, although most metabolites are subject to renal clearance. The metabolites of gliclazide and glipizide are inert or only weakly active, so these particular sulfonylureas can be used even in patients with ESRD receiving dialysis. Their use does carry the risk of hypoglycemia, especially as GFR declines and insulin clearance decreases. Sulfonylureas are highly protein-bound but can be displaced into circulation by other drugs used in diabetic patients (e.g., salicylates, β-blockers, fibric acid derivatives), further contributing to hypoglycemia.
Thiazolidinediones
The thiazolidinediones are peroxisome proliferator-activated receptor (PPAR) modulators, which work to increase insulin sensitivity. Their use is limited by resultant weight gain and fluid retention through transcriptional upregulation of amiloride-sensitive sodium (Na+) channels in renal tubules, which is problematic in a population already prone to CVD and heart failure. Rosiglitazone was withdrawn from the market because of the suggestion of an increased risk of myocardial infarction with its use, although pioglitazone remains in use.
Meglinitides
The main drugs in the meglinitide class, nateglinide and repaglinide, are primarily metabolized in the liver and act as insulin secretagogues. Repaglinide is safe to use in diabetic patients with advancing renal failure because it is converted to inactive metabolites and mainly excreted in bile, with less than 10% of the parent drug appearing in the urine, so no dose adjustments are deemed necessary. More than 80% of nateglinide is excreted in the urine, and thus it should only be used cautiously, if at all, in advanced CKD.
Glucagon-like Peptide-1 Analogues
Glucagon-like peptide-1 (GLP-1) analogues such as exenatide and liraglutide work by enhancing glucose-mediated insulin secretion in response to food entering the gut and maintaining the integrity of insulin-producing beta cells. The GLP-1 analogues help stimulate weight loss by appetite suppression, both centrally and by affecting gastric motility. Exenatide and liraglutide are not recommended if GFR falls below 30 and 60 ml/min/1.73 m2, respectively.
Gliptins
The gliptin class inhibits the effect of dipeptidyl peptidase-4 (DPP4), a cellular membrane protein expressed in a variety of tissues whose function is to rapidly degrade incretin hormones such as GLP-1. Sitagliptin is excreted mostly unchanged (70% to 80%) in the urine, requiring dose reductions as GFR drops below 50 ml/min/1.73 m2 (50% reduction) and 30 ml/min/1.73 m2 (75%). Newer agents such as linagliptin are primarily metabolized by the liver and excreted in the bile, not needing dose reduction in CKD. The gliptins have the advantage of being weight neutral, unlike insulin secretagogues.
Insulin
Exogenous insulin, unlike endogenously secreted insulin, which undergoes first-pass metabolism in the liver, is primarily eliminated by the kidneys through free filtration and secretion into the renal tubules, before reuptake and degradation by peritubular cells. In terms of endogenous insulin handling, the kidneys extract up to 40% of all plasma insulin down to GFR of 20 ml/min/1.73 m2, with less than 10% extraction at ESRD.8
As GFR falls below 60 ml/min/1.73 m2, insulin requirements in both type 1 and type 2 diabetic patients progressively fall, by up to 40% to 50%, regardless of residual insulin secretion in type 2 patients. This decline is especially marked as GFR falls below 20 ml/min/1.73 m2 and approaches ESRD.9 This helps to explain the phenomenon of hypoglycemia in nondiabetic patients with advanced CKD, although importantly, the kidney is an important site for gluconeogenesis, which can fail as CKD progresses.
Insulin resistance itself results from CKD. Uremia induces insulin resistance, thought to be caused by a defect in post–receptor signaling, with reduced glucose uptake and metabolism after insulin receptor activation peripherally. In skeletal muscle tissue, insulin-mediated glucose metabolism is mediated through phosphatidylinositol-3 kinase (PI3K-AKt) secondary messenger pathways, which are adversely affected by many factors associated with renal impairment, such as metabolic acidosis, uremia, anemia, elevated levels of proinflammatory cytokines, and low vitamin D levels.
In terms of the types of insulin used, available data favor the use of analogues, where the insulin molecule has been modified for both rapid-acting and long-acting forms so as to possess pharmacokinetics more in keeping with physiologic insulin secretion, in contrast to traditional human insulins. In a comparative study of insulin lispro versus human insulin in diabetic patients on hemodialysis, insulin was injected just after starting a 4-hour dialysis session. Insulin lispro was shown to have a faster rate of absorption with a shorter time to peak concentration (which was also of greater overall magnitude) and elimination.10
Another study on the pharmacodynamics and pharmacokinetics of insulin lispro versus human insulin in type 1 diabetic patients found that both attained a higher peak concentration and prolonged clearance in patients with overt DN, with an average reduction of insulin clearance by 30% to 40%.11 However, despite higher circulating levels, human insulin was found to have a paradoxically lower peak effect with reduced overall metabolic activity in diabetic patients with DN than in those with normal GFR. Insulin lispro maintained a similar metabolic profile, regardless of whether DN was present. Insulin aspart, another rapid-acting analogue, has also been found to have a metabolic profile largely unaffected by various stages of CKD, along with long-acting analogues such as glargine and detemir.
Although there is no formal insulin regimen recommended in the setting of CKD, for type 1 diabetic patients, the basal bolus regimen of 3 daily injections of short-acting insulin with meals combined with 1 or 2 injections of long-acting insulin, as used in DCCT, is the standard treatment regimen, with the added benefit of greater flexibility over a regimen with fewer injections. Type 2 diabetic patients, when requiring insulin, usually start on once-daily or twice-daily, long-acting or intermediate-acting insulin, if necessary moving onto mixed formulations (fixed percentages of short-acting and longer-acting insulins) or the basal bolus regimen as in type 1 patients. Again, as CKD progresses, this may need regular adjustment.
Hypertension
The most common blood pressure (BP) target, set by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) and other major societies, was a BP below 130/80 mm Hg for patients with CKD caused by diabetes mellitus,12 with the cutoff derived from observational studies such as the MRFIT study and the Okinawa Screening Programme, with risk of developing ESRD beginning to increase significantly beyond the 130/80 mm Hg target (see Chapter 80).
Despite this, there is a lack of strong evidence from randomized controlled trials (RCTs) to support this target in secondary prevention of renal disease, with systemic review of relevant trials showing no benefit for tight BP control on renal outcomes such as the rate of decline of GFR and progression to ESRD.13 Some benefit was found in patients with frank proteinuria, but this required more antihypertensive agents, with a slightly increased risk of adverse events. This must be taken with the caveat that the numbers of patients involved were quite small and the trials short in duration, possibly limiting their ability to detect differences.
The recent Kidney Disease: Improving Global Outcomes (KDIGO) guidelines on managing hypertension in CKD patients with diabetes reflects this information, with a more conservative target of 140/90 mm Hg or less in patients with GFR below 60 ml/min/1.73 m2,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree