Management of Overactive Bladder
Joseph M. Montella
DEFINITION
As defined by the International Continence Society, overactive bladder (OAB) is the condition in which a patient has symptoms of urgency with or without urge incontinence, usually with frequency and nocturia, in the absence of infection, metabolic disturbance, or other pathologic factors that would account for these symptoms. It is a symptomatic diagnosis and therefore does not require the performance of urodynamic testing or cystometry for confirmation. Urgency is defined as the feeling that the patient must void immediately for fear of losing urine, and frequency is defined as greater than 10 micturitions in a 24-hour period. Urge incontinence describes involuntary loss of urine associated with an urgent, strong desire to void.
The term “detrusor overactivity” (unstable bladder) is more restrictive and describes an OAB caused by detrusor contractions documented by cystometrogram. Detrusor overactivity occurs when the bladder contracts spontaneously, or on provocation, during bladder filling while the patient is attempting to inhibit micturition. Detrusor overactivity is diagnosed during provocative cystometry when one of the following conditions occurs: a true detrusor pressure rise of 15 cmH2O (motor urge incontinence) or a true detrusor pressure rise of less than 15 cmH2O in the presence of urgency or urge incontinence (sensory urge incontinence) (1). Subthreshold detrusor contractions of less than 15 cm H2O may have clinical significance and have been shown to cause urinary incontinence in 10% and urgency in 85% of patients (2).
Additionally, a urodynamic diagnosis associated with the symptom of urge incontinence in a frail elderly patient is detrusor hyperactivity with impaired contractility (DHIC). These patients have involuntary detrusor contractions causing incontinence but are unable to empty their bladders completely, leaving a large postvoid residual (3). A pressure rise during filling may represent decreased bladder compliance or insufficient time to accommodate the increase in volume because cystometry is time dependent (4), and this would not be considered as detrusor overactivity in this context.
Detrusor hyperreflexia is detrusor overactivity secondary to a known neurologic abnormality (1). The term “neurogenic bladder” is reserved for spinal cord injuries and other similar defects and their impact on bladder function.
Incorrect synonyms that have been applied to OAB include bladder dyssynergia and vesical instability. These terms should no longer be used.
PREVALENCE AND IMPACT ON QUALITY OF LIFE
The National Overactive Bladder Evaluation (NOBLE) program estimated the overall prevalence of OAB as 16.9% of women and 16.0% of men, a rate that corresponds to 33.3 million adult Americans, with an impact on quality of life equal to that of urinary incontinence (5,6). The occurrence of involuntary detrusor contractions in infancy is a normal state for bladder emptying and is later controlled by the development of cortical inhibition of reflex bladder activity. Farrar et al (7) described the prevalence of OAB as 8% to 50%, depending on age distribution. In more than 2,000 women studied by Abrams (8), OAB occurred in 38% of those 65 years of age or older and in 27% of those younger than 65 years of age. In institutionalized women, the incidence of urinary incontinence secondary to OAB is greater than 80% (9). Thus, the prevalence of OAB is greatest at the extremes of life; OAB has a 5% to 10% occurrence in premenopausal patients,
increasing to as much as 38% in elderly patients and perhaps to more than 80% in institutionalized incontinent elderly patients.
increasing to as much as 38% in elderly patients and perhaps to more than 80% in institutionalized incontinent elderly patients.
Although the severity of OAB has been measured by outcome variables such as micturition frequency or quantity of urine lost, the impact on quality of life must also be measured in terms of physical and psychological functioning. Psychosocial complications included disturbed sleep, impaired mobility and work productivity, isolation and depression, impaired domestic and sexual functioning, and diminished quality of life (10).
CLINICAL PRESENTATION
The symptoms of OAB include urgency, frequency (greater than 10 micturitions in a 24-hour period), urge incontinence, and nocturia (two times or more). There can also be a history of childhood nocturnal enuresis in some patients (11). OAB may coexist with genuine stress incontinence, and stressful activity may trigger a detrusor contraction causing urge incontinence. In 100 women with the urodynamic diagnosis of detrusor overactivity, Wiskind et al (12) reported that although 86% of patients had symptoms of urge incontinence, 76% also complained of stress incontinence. Sand et al (13) reported on 188 incontinent women, and of those reporting only stress incontinence, 34.9% had detrusor overactivity. Only 32.6% of patients reporting both urge and stress incontinence had detrusor overactivity.
DIFFERENTIAL DIAGNOSIS
Because the symptoms of OAB overlap with those of other lower urinary tract conditions, a number of other diagnoses must be entertained. Table 11.1 lists the differential diagnosis for these symptoms. A special word must be written about urethral instability, which tends to be rather poorly defined. Wise et al (14) investigated the prevalence and significance of urethral instability in a group of women with OAB. This occurred in 42% of patients with OAB and was strongly associated with the sequence of relaxation of the urethra before unprovoked detrusor contraction. Women with OAB and a stable urethra exhibited primary contraction of the detrusor, whereas the symptom of stress incontinence was more common in women with urethral instability. The investigators postulated that women with OAB should be divided into two groups: those with and those without urethral instability, the latter group possibly benefiting from α-agonist therapy. In addition, Petros and Ulmsten (15) found that provocative urethrocystometry revealed a rise in detrusor pressure followed by a fall in urethral pressure, both preceded by urge symptoms. They concluded that urethral instability, OAB, and urge incontinence were different manifestations of a prematurely activated micturition reflex. Urethral instability may not be a separate entity but a part of urine loss associated with urge.
TABLE 11.1 Differential Diagnosis of OAB | ||||||||
|---|---|---|---|---|---|---|---|---|
|
PATHOPHYSIOLOGY
Table 11.2 lists the etiologies of OAB. Neurologic diseases (multiple sclerosis, cerebrovascular disease, parkinsonism, Alzheimer’s disease), local bladder and urethral irritants (cystitis, foreign bodies, tumors), outflow obstruction (severe cystocele or vaginal vault prolapse), and medications (parasympathomimetics) must be considered as etiologies. Most cases, however, apart from those in very young or elderly patients, are idiopathic in nature. Del Carro et al (16) compared women with idiopathic OAB with age-matched controls using subtracted cystometry and anal sphincter electromyography sacral reflex analysis along with other neurologic tests using evoked potentials. All patients had normal neurophysiologic tests, and there was no significant difference between patients and controls. Because women with OAB do not appear to have either clinical or subclinical damage of central sensory or motor pathways, other investigators have put forth their theories regarding intrinsic bladder abnormalities. The pathophysiology of OAB may be principally neurogenic, myogenic, obstructive, or idiopathic.
Neurogenic
The bladder is never really in a complete resting state. Rather, in vitro and in vivo studies show that
it is in continuous activity, with rhythmic contractions that wax and wane (17,18). Van Duyl (19) suggested that small regional contractions from possible pacemaker cells might be the origin of large bladder contractions. The sacral parasympathomimetics that originate from S2 to S4 are the major excitatory input to the urinary bladder. The corresponding ganglia lie within the bladder itself. In childhood, involuntary spontaneous and rhythmic contractions occur, but these are eventually suppressed with the maturation of cortical control. Normal human bladder contractions are primarily mediated by acetylcholine released from cholinergic nerve terminals in the bladder. Vasoactive intestinal polypeptide (VIP), a neuropeptide, has been found to be present in a certain proportion of cholinergic ganglion cells and functions as an inhibitory agent in this parasympathomimetic pathway. Furthermore, VIP is noted to be in reduced concentrations in detrusor muscles of patients with OAB (20).
it is in continuous activity, with rhythmic contractions that wax and wane (17,18). Van Duyl (19) suggested that small regional contractions from possible pacemaker cells might be the origin of large bladder contractions. The sacral parasympathomimetics that originate from S2 to S4 are the major excitatory input to the urinary bladder. The corresponding ganglia lie within the bladder itself. In childhood, involuntary spontaneous and rhythmic contractions occur, but these are eventually suppressed with the maturation of cortical control. Normal human bladder contractions are primarily mediated by acetylcholine released from cholinergic nerve terminals in the bladder. Vasoactive intestinal polypeptide (VIP), a neuropeptide, has been found to be present in a certain proportion of cholinergic ganglion cells and functions as an inhibitory agent in this parasympathomimetic pathway. Furthermore, VIP is noted to be in reduced concentrations in detrusor muscles of patients with OAB (20).
TABLE 11.2 Etiologies of OAB | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The persistence or reappearance of such uncontrolled contractions is possibly related to an aberrant control mechanism. This suggests a disorder of the intrinsic neuromodulatory mechanism leading to OAB. This can result from the loss of suprapontine inhibition from conditions such as cerebrovascular disease, Alzheimer’s disease, or Parkinson’s disease or from facilitation of excitatory influences. Neurotransmitters involved in these conditions include the glutamatergic, dopaminergic, cholinergic, GABA-ergic (21), and serotonergic systems (22).
Sensory afferents also play a role in bladder control. The interruption of descending inhibitory pathways leads to a reorganization of afferent pathways, with unmyelinated C-fibers becoming prominent in the micturition arc. Sensitization of these fibers increases bladder excitability and lowers the threshold for pain (23), leading to OAB.
Myogenic
Enhanced, spontaneous contractile activity of the detrusor has been noticed. In vitro studies by Kinder and Mundy (17) showed that muscles from bladders with OAB, regardless of the etiology, spontaneously contract more often and with a greater amplitude than muscles from urodynamically normal bladders. Structural abnormalities have been noted in both neuropathic and nonneuropathic unstable detrusor muscles in the form of narrower junctional gaps between muscle cells as compared with the normal detrusor (24). A significant proportion of patients with irritable bowel syndrome have urinary complaints, including urgency and nocturia. Whorwell et al (25) studied such patients urodynamically and found that 50% of these patients have OAB. They suggested that this high incidence of OAB is secondary to a diffuse disorder of smooth muscle or its innervation.
Obstructive
It has been thought that outflow obstruction in the male with prostatic hypertrophy is associated with OAB because the relief of this obstruction usually leads to the resolution of OAB. However, Abrams (8) has shown that OAB may be related to advanced age and that a postoperative decrease in instability may be due to interruption of sensory afferents. Obstruction with high outflow pressure in women is rare. Abrams studied more than 2,000 female patients and found that only 3.7% had outlet obstruction, defined by a maximum flow rate of less than 15 m/s. Additionally, there was no increased incidence of OAB in those patients with outflow obstruction.
It may be hypothesized that elevation of the vesical neck by surgical repair for stress incontinence leads to excessive urethral compression and
could cause outflow obstruction, resulting in OAB. However, this is not correlated with changes in peak flow rates and maximum voiding pressures. Furthermore, patients with vaginal prolapse and pre-existing OAB are not usually cured of their OAB by repairing the prolapse (26).
could cause outflow obstruction, resulting in OAB. However, this is not correlated with changes in peak flow rates and maximum voiding pressures. Furthermore, patients with vaginal prolapse and pre-existing OAB are not usually cured of their OAB by repairing the prolapse (26).
The incidence of de novo OAB in patients who preoperatively have only genuine stress urinary incontinence ranges from 5% to 18% (27,28). Cardozo et al (27) postulated that repeat surgeries at the vesical neck interfere with the autonomic nerve supply of the bladder and result in OAB. In a review of six studies of patients who had a Burch colposuspension performed for stress incontinence, Vierhout and Mulder (29) found a prevalence of between 5% and 27%, with 68 of 396 patients developing de novo OAB.
DIAGNOSIS
All patients with OAB symptoms should undergo a basic evaluation, as outlined in the guidelines proposed by the Agency on Health Care Policy and Research (30), that includes a history, physical examination, measurement of postvoid residual volume, and urinalysis. Any risk factors that are associated with urinary incontinence should be identified and attempts made to modify them.
The classic history of OAB is that of a strong urge to void or a voiding frequency of greater than 10 micturitions in a 24-hour period that can be associated with sudden urine loss. The history should also include the following elements:
A focused medical, neurologic, and genitourinary history that includes an assessment of risk factors and a review of medications
A detailed exploration of the OAB symptoms, including duration
Quality-of-life assessment
Associated symptoms, such as stress incontinence and pelvic organ prolapse
Fluid intake pattern by using a 24- to 72-hour voiding diary
Number of pads used
Previous treatments and their success
Expectations for outcomes of treatment
Assessment of mobility, living environment, and social factors
The physical examination should include the following:
Neurologic evaluation of the lower sacral segments, including bulbocavernosus and anal wink reflexes
Mental status examination
Abdominal examination to evaluate for masses or fluid collections, which may influence intra-abdominal pressure and detrusor physiology
Pelvic examination, which usually reveals normal support in patients with OAB; however, severe genitourinary prolapse, hypoestrogenism, and urethral diverticulum must be ruled out because these conditions may contribute to the OAB symptoms. A rectal examination can determine sphincter tone, fecal impaction, or rectal mass.
Cough stress test to determine the presence of stress incontinence. Although the presence of stress incontinence does not rule out OAB, it can affect the treatment outcomes.
Estimation of postvoid residual volume either by catheterization or pelvic ultrasound. Residuals of less than 50 mL are considered normal. Repetitive postvoid residuals ranging from 100 to 200 mL or higher are considered incomplete bladder emptying. Postvoid residual determination is important to document adequate detrusor function and rule out DHIC. Urinalysis and culture are used to rule out hematuria (which may be indicative of a tumor or stone in the urinary tract), glucosuria (which may cause increased voiding frequency), pyuria, and bacteriuria. After any correctable problems (e.g., hematuria, infection) are identified and solved, therapy can be directed toward treating the OAB.
Advanced Testing
Advanced testing for OAB can be employed in the following situations:
Failure of the patient to respond to an adequate therapeutic intervention
Hematuria without infection
Persistent voiding dysfunction
Symptomatic genitourinary prolapse
Uncertain diagnosis from the basic evaluation
Uroflowmetry may reveal obstructive voiding patterns secondary to severe genitourinary prolapse or tumor.
If urodynamic testing is to be employed, it is also important to duplicate closely the circumstances surrounding urine loss, which would include provocative maneuvers such as coughing, positional changes, running water, hand washing, rapid filling, and temperature change of the filling medium. These can increase the sensitivity of the test (Fig. 11.1). Without provocation, detrusor overactivity will go undiagnosed in 30% to 40% of patients (12).
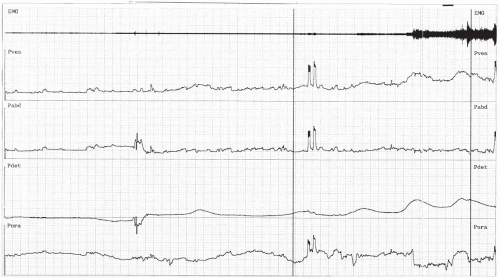 FIGURE 11.1 ● Multichannel cystometrogram illustrating detrusor instability. The patient had a detrusor contraction after she washed her hands. Before provocation, she had no contraction. |
In cases in which traditional cystometry fails to produce a diagnosis, alternative methods may be used. One such method is extramural ambulatory urodynamic monitoring. McInerney et al (31) and Webb et al (32), in two separate studies, pronounced ambulatory monitoring as more sensitive in the diagnosis of detrusor overactivity than conventional cystometry. Porru and Usai (33) used this technique in 46 patients with urinary incontinence, 16 of whom had urge incontinence symptoms. Conventional cystometry identified detrusor contractions in only 50% of these patients, whereas ambulatory monitoring identified detrusor contractions in 93%.
Another technique involves diuresis cystometry, in which a patient is given a diuretic to fill the bladder to approximate more closely the anterograde filling phase. Van Venrooij and Boon (34) evaluated women with frequency and urge incontinence with a negative retrograde cystometrogram using diuresis cystometry and noted an increase in the detection of detrusor overactivity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








