Chapter 37 Malignant Biliary Obstruction of the Hilum and Proximal Bile Ducts
Malignant biliary obstruction of the hilum and proximal intrahepatic bile ducts can result from primary pancreatobiliary cancers, primary liver cancers, portal lymphadenopathy, or metastatic disease. Primary pancreatobiliary cancers affecting the proximal bile ducts and hilum include cholangiocarcinomas and gallbladder cancer. Cholangiocarcinomas can cause obstruction at any level of the biliary tract. Cancer of the gallbladder can present with hilar or right intrahepatic duct obstruction due to local tumor extension or extrinsic compression from portal adenopathy or Mirizzi syndrome. This chapter will primarily focus on the diagnosis and management of hilar tumors. Refer to Table 37.1 for differential diagnosis of hilar strictures.1–3 Malignant biliary obstruction of the distal bile ducts (including distal cholangiocarcinoma) is discussed in Chapter 36.
Table 37.1 Differential Diagnosis of Hilar Strictures
| Malignant | Benign |
|---|---|
| Cholangiocarcinoma | Sclerosing cholangitis |
| Gallbladder cancer | Choledocholithiasis/hepatolithiasis |
| Nodal metastases at porta hepatis | Inflammatory strictures |
| Hepatocellular carcinoma | Postoperative |
| Hepatic metastases | Extrinsic compression (Mirizzi syndrome) |
| Metastases to biliary tree | Benign fibrosing/sclerosing cholangitis |
| Postradiation | |
| Caroli disease | |
| Ischemia | |
| Infection |
Data from Wetter LA, Ring EJ, Pellegrini CA, et al. Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg. 1991;161(1):57. Verbeek PC, van Leeuwen DJ, de Wit LT, et al. Benign fibrosing disease at the hepatic confluence mimicking Klatskin tumors. Surgery. 1992;112(5):866. And Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660.
Cholangiocarcinoma
Cholangiocarcinomas arise from epithelial cells of the bile ducts. They account for approximately 3% of all gastrointestinal malignancies. Cholangiocarcinomas can be divided into proximal (intrahepatic), hilar, or distal (extrahepatic) cancers. Cancers arising in the hilar region have been further classified according to the pattern of involvement of the hepatic ducts (see discussion below on Bismuth-Corlette classification). Perihilar tumors account for approximately 60% to 70% of all cholangiocarcinomas, while distal and mid bile duct tumors account for 30% to 40% and those arising from the proximal intrahepatic ducts account for less than 10%.4
Risk factors for the development of cholangiocarcinoma are related to chronic inflammation and infection. Almost one third of cholangiocarcinomas are diagnosed in patients with primary sclerosing cholangitis (PSC) with or without associated ulcerative colitis.5 Other risk factors for cholangiocarcinoma include polycystic liver disease, parasitic infection, hepatolithiasis, toxic exposures (such as thorotrast and rubber), and genetic disorders (such as Lynch syndrome and biliary papillomatosis).6–8
The majority of cholangiocarcinomas (>90%) are adenocarcinomas. Squamous cell carcinomas account for most of the remaining cases. Adenocarcinomas are divided into nodular, sclerosing, and papillary types. Both the nodular and the sclerosing (scirrhous) tumors have lower resection and cure rates.9
Anatomy of Bile Ducts
Segmental Liver Anatomy
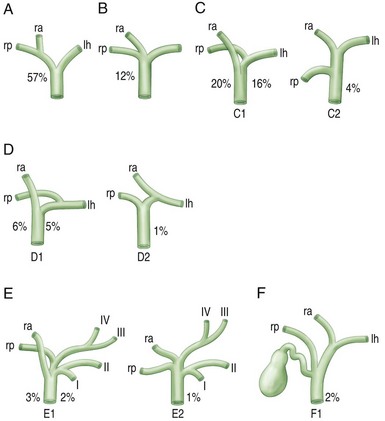
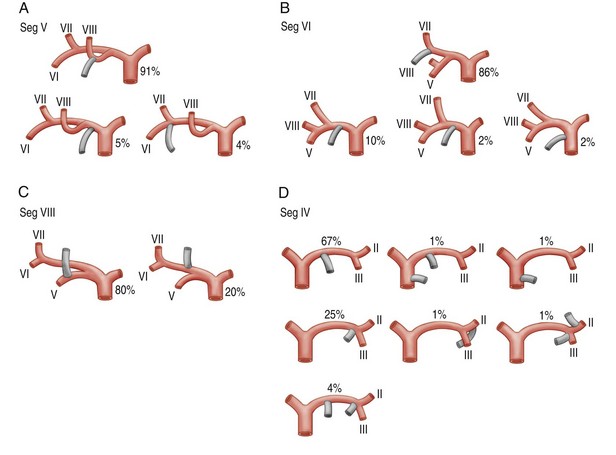
It is a common misperception that the bile ducts are simply shaped like a “Y,” with the right and left ducts joining together to form the common hepatic duct. In reality, the anatomic pattern of the biliary tree can be quite variable and includes eight segments (Fig. 37.1; see also Chapter 32). Knowledge of this anatomy, as well as the common variations of the segmental intrahepatic ducts and biliary confluence (Figs. 37.2 and 37.3), is essential to successful endoscopic management of complex hilar cholangiocarcinoma. Segment I (caudate lobe) is typically drained by several small ducts into both the right and the left ductal systems. These branches are generally not seen on ERCP. Segments II, III, and IV comprise the left lobe. Segment II/III is usually the large left intrahepatic duct that is targeted on endoscopic therapy. Segment IV is further divided into two smaller segments, IVa and IVb, which are typically not targets of endoscopic drainage given the small portion of hepatic parenchyma they drain. The right hepatic ducts are divided into the right anterior sectoral duct, which drains segments V and VIII, and the right posterior sectoral duct, which drains segments VI and VII.10,11
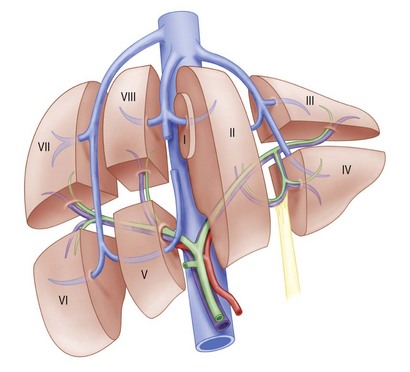
Fig. 37.1 Functional division of the liver into segments, according to Couinaud’s nomenclature.
(Redrawn from Blumgart LH, Fong Y, eds: Surgery of the liver and biliary tract. 3rd ed. Philadelphia: W.B. Saunders; 2000.)
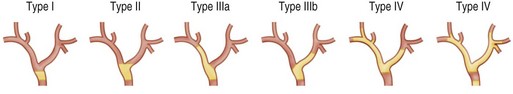
Bismuth-Corlette Classification12
Cancers arising in the perihilar region have been classified according to their pattern of involvement of the hepatic ducts. The Bismuth classification for cholangiocarcinoma is useful for determining and planning surgical resection as well as endoscopic stent placement. Bile duct tumors that involve the confluence of the major ducts are referred to as Klatskin tumors or hilar cholangiocarcinomas (Fig. 37.4).
 Type I: Tumors below the confluence of the left and right hepatic ducts
Type I: Tumors below the confluence of the left and right hepatic ducts
 Type II: Tumors reaching the confluence of the right and left hepatic ducts
Type II: Tumors reaching the confluence of the right and left hepatic ducts
 IIIa: Tumors occluding the common hepatic duct and the first radicals of the right intrahepatic system
IIIa: Tumors occluding the common hepatic duct and the first radicals of the right intrahepatic system IIIb: Tumors occluding the common hepatic duct and the first radicals of the left intrahepatic system
IIIb: Tumors occluding the common hepatic duct and the first radicals of the left intrahepatic system Type IV: Tumors that are multicentric or that involve the confluence of the major ducts and radicals of both right and left intrahepatic ducts
Type IV: Tumors that are multicentric or that involve the confluence of the major ducts and radicals of both right and left intrahepatic ducts
Clinical Presentation
More advanced biliary obstruction often presents as fatigue, anorexia, and painless jaundice. Other common symptoms include pruritus (66%), abdominal pain (30% to 50%), weight loss (30% to 50%), and fever (10% to 20%).6
Diagnostic Evaluation in Patients with Hilar and Proximal Biliary Obstruction
Laboratory Studies
Routine Blood Work
Although nonspecific, serum liver chemistry is usually consistent with a pattern suggestive of biliary obstruction; the degree of elevation depends on the location, severity, and chronicity of the obstruction. A proximal lesion can be associated with an isolated alkaline phosphatase elevation. A prolonged prothrombin time may be seen in patients with chronic biliary obstruction.13
Tumor Markers
CEA: Serum CEA alone is neither sufficiently sensitive nor sufficiently specific to diagnose cholangiocarcinoma. Elevations in CEA can be seen with benign conditions such as gastritis, peptic ulcer disease, diverticulitis, liver disease, chronic obstructive pulmonary disease, diabetes mellitus, and other acute and chronic inflammatory states.14,15
CA 19-9: The role of serum levels of CA 19-9 have been widely studied for detection of cholangiocarcinoma, particularly in the setting of PSC. The limitations of this test include its low sensitivity and specificity. CA 19-9 is often elevated in patients with various pancreatobiliary disorders, including pancreatitis, cholangitis, pancreatic cancer, and other malignancies. In addition, biliary obstruction of any etiology can result in elevated serum CA 19-9 levels.16
Radiographic Evaluation
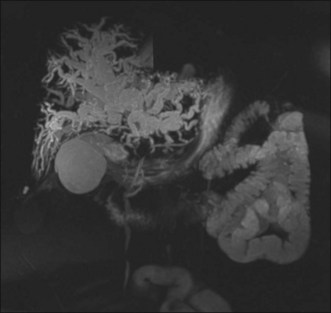
In a patient with painless jaundice, computed tomography (CT) scan and magnetic resonance imaging (MRI) are the preferred imaging modalities. For hilar and intrahepatic lesions, MRI with dedicated liver protocol and magnetic resonance cholangiopancreatography (MRCP) is the imaging test of choice. MRCP can create three-dimensional images of the biliary tree, aiding in anatomic visualization and procedure planning (Fig. 37.5).17,18 Unfortunately, availability and expertise of MRI is center dependent. Contrast-enhanced CT scans are more readily available and are also an excellent imaging tool. Positron emission tomography (PET scan) has been shown to detect nodular cholangiocarcinomas as small as 1 cm but is less helpful for detection of infiltrating tumors, and its sensitivity is also dependent on local expertise. Studies using PET scans have demonstrated variable success rates in identifying otherwise unsuspected distant metastatic disease.19
Endoscopic Evaluation
EUS
While endoscopic ultrasound (EUS) with fine-needle aspiration (FNA) can be performed to evaluate hilar lesions, it is more challenging than for distal common bile duct (CBD) and pancreatic lesions.20 In addition, EUS with FNA can be performed to evaluate and sample portal adenopathy and accessible lesions in the liver (particularly the left lobe).21,22 The potential advantage of EUS over ERCP is less invasiveness and it provides information in patients who do not otherwise require biliary drainage. One concern, however, is the potential for tumor seeding from the needle tract during EUS-FNA,23,24 a particular concern in patients who are candidates for curative surgical resection.
ERCP
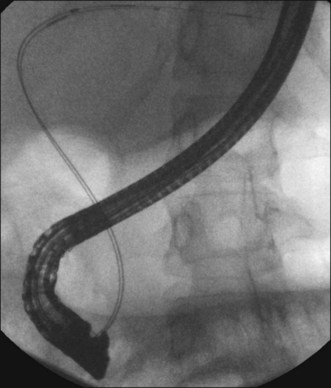
ERCP is the preferred method for obtaining tissue diagnosis and providing biliary drainage (Fig. 37.6). Additional tools that can enhance inspection of strictures during ERCP include cholangioscopy (see also Chapter 26), confocal imaging, intraductal ultrasound (IDUS), narrow band imaging (NBI), and chromoendoscopy. Maximal effort at visual and histopathologic diagnosis of malignancy should be attempted at the time of initial ERCP, since manipulation and trauma from catheters and stent placement can affect interpretation of results during subsequent procedures.
Intraductal Ultrasound (IDUS)
IDUS probes are approximately 2 mm in diameter and can be advanced over a guidewire without need for sphincterotomy. IDUS can help determine the longitudinal tumor extent more accurately than cholangiography. IDUS can also be used to evaluate for tumor invasion into the portal vein and right hepatic artery.25,26 With the advent of more user-friendly cholangioscopy systems, IDUS is performed less frequently.
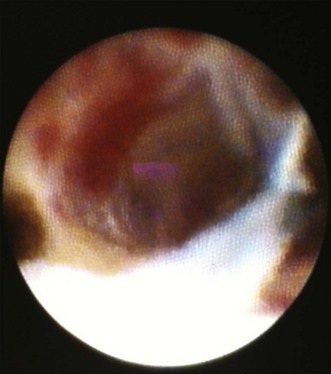
Cholangioscopy
Of the above mentioned tools, cholangioscopy (Fig. 37.7) is most frequently used given the ability to visualize ducts, allowing for further characterization of strictures and direct targeting of biopsies.27–29 Also, more current single-operator systems (SpyGlass, Boston Scientific, Natick, Mass.) are technically easier to use,30 though the fiberoptic images may have lower resolution than those obtained with video chips. Cholangioscopy is discussed in more detail in Chapter 26.
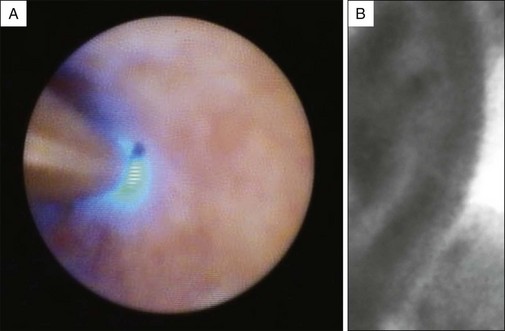
Confocal Laser Endomicroscopy (CLE)
More recently confocal imaging has been added to the endoscopic armamentarium. Confocal laser endomicroscopy (CLE) illuminates tissues with a low-power laser and detects reflected fluorescent light, eliminating scattered light and increasing spatial resolution. Intravenous fluorescein is used to highlight the vasculature, lamina propria, and intracellular spaces of examined tissues. The confocal probe (CholangioFlex probe, Mauna Kea Technologies, Paris) has a diameter of 0.9 mm and can be advanced through the instrument channel of a cholangioscope or catheter (Fig. 37.8).26 A multicenter trial using probe-based confocal laser endomicroscopy (pCLE) found a significantly higher accuracy when the combination of ERCP and pCLE was used compared with ERCP and tissue acquisition (90% versus 73%). The sensitivity, specificity, positive predictive value, and negative predictive value of pCLE for detecting cancerous strictures were 98%, 67%, 71%, and 97%, respectively, compared with 45%, 100%, 100%, and 69%, respectively, for routine pathology. One major limitation, however, was that the investigators were not blinded to the clinical information, which could potentially lead to bias. Further studies are needed to better evaluate the role of pCLE in the evaluation of bile duct strictures.31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree