 LEFT VENTRICULAR HYPERTROPHY AND HEART FAILURE
LEFT VENTRICULAR HYPERTROPHY AND HEART FAILURE
Pathogenesis of Left Ventricular Disorders
Ventricular growth occurs in response to mechanical stresses, primarily volume or pressure overload (6). Volume overload results in addition of new sarcomeres in series, leading to increased cavity diameter (7). Larger diameter results in increased wall tension, a direct consequence of Laplace’s law. This increase in wall tension stimulates the addition of new sarcomeres in parallel. Such remodeling thickens the ventricular wall, distributing the tension over a larger cross-sectional area of muscle and returning the tension in each individual fiber back toward normal. This combination of cavity enlargement and wall thickening is called eccentric hypertrophy. Pressure overload, on the other hand, increases wall tension by increasing intraventricular pressure, resulting directly in the parallel addition of new sarcomeres. Because sarcomeres are not added in series, isolated pressure overload leads to concentric hypertrophy, that is, wall thickening without cavity enlargement.
Both eccentric and concentric hypertrophy are initially compensatory and therefore beneficial. Dilatation permits an increase in stroke volume without an increase in the inotropic state of the myocardium and as such is an efficient adaptation to volume overload (8). It also permits the maintenance of a normal stroke volume and cardiac output in the presence of decreased contractility. Muscular hypertrophy returns the tension per unit muscle fiber back to normal, decreasing ventricular stress.
If the stimuli for ventricular remodeling persist, however, LVH eventually becomes maladaptive. Hypertrophy is associated with progressive, deleterious changes in myocardial cells. Early in the evolution of LVH, abnormalities of cellular calcium handling lead to abnormal ventricular relaxation; combined with decreased passive compliance of a thickened ventricular wall, these changes may precipitate diastolic dysfunction (9). Decreased capillary density, impaired coronary reserves, and abnormal relaxation may decrease subendocardial perfusion, promoting ischemia (10). Frequent coexistence of coronary artery disease (CAD) may exacerbate the situation. Fibrosis of the cardiac interstitium also occurs and appears to be more marked in pressure than volume overload (11). In the late phases of chronic overload, oxidative stress is prominent and contributes to cellular dysfunction and demise (12). Together, these various processes lead to progressive cellular attrition, fibrosis, HF, and death. The results of the 2013 Chronic Renal Insufficiency Cohort (CRIC) study are consistent with the HF literature, whereby LVH is a precursor to systolic dysfunction (13). A subset of 190 patients from this longitudinal study had serial echocardiograms during advanced CKD [mean estimated glomerular filtration rate (eGFR) 16.9 mL/min/1.73 m2] and ESKD. The majority (80%) of patients included had LVH at baseline, and the left ventricular mass index (LVMI) did not change significantly from advanced CKD to ESKD suggesting LVH is fixed by moderate to advanced stages of CKD. There was a significant decline in ejection fraction, however, from 53% to 50% (p = 0.002).
Many factors unique to patients with CKD appear to contribute to cardiac dysfunction. Anemia, salt and water overload, and arteriovenous (AV) fistulas in hemodialysis patients are common causes of volume overload. Hypertension is highly prevalent in patients with ESKD and is a major cause of pressure overload. These same factors promote arterial remodeling in the large and resistance arteries, characterized by diffuse arterial thickening and stiffening (arteriosclerosis), which can increase the effective load on the LV independently of mean arterial pressure (12,14).
Aside from hemodynamic factors, the uremic milieu may also lead to myocyte death. Although CAD is the major factor promoting ischemia and infarction, hyperparathyroidism increases susceptibility to ischemia through dysregulation of cellular energy metabolism (15). Poor nutrition, oxidative stress, and inadequate dialysis may all additionally promote myocyte death (6,16,17).
Diagnosis of Left Ventricular Disorders in Dialysis Patients
LV disorders can be asymptomatic or they may be manifested clinically as HF, arrhythmias, dialysis-associated hypotension, or ischemic symptoms. The diagnosis of HF is based on clinical symptoms and signs and can usually be made with an appropriate history and physical examination. HF typically presents as progressive fatigue and a decline in exercise tolerance or, alternatively, as a syndrome characterized by dyspnea, jugular venous distension, and bilateral lung crepitations and the characteristic chest x-ray appearance.
Some physicians attempt to distinguish HF from “volume overload” or circulatory congestion. This is a difficult task, as the clinical findings of both conditions are identical. The clinical context may be of some assistance, especially when fluid gains are known to be minimal based on the patient’s dry weight. Even in this situation, diastolic dysfunction may be the underlying problem. In general, HF symptoms are not likely to occur in a patient with a perfectly normal heart. Physicians are well advised to consider all episodes of symptomatic HF as evidence of some degree of myocardial dysfunction and investigate them further.
Echocardiography is an important method for the assessment of LV structure and function (18–20). Although LV mass measurement using echocardiography is highly reproducible between observers, its measurement varies over the course of a hemodialysis session by as much as 25 g/m2 (20). This occurs because LV internal diastolic diameter decreases as the patient’s blood volume decreases, as a result of fluid withdrawal. A concomitant decrease in LV wall thickness is not observed. Consequently, the LVMI measured predialysis is higher than the postdialysis measurement, although the actual LV mass has not changed. Therefore, when possible, imaging should be carried out when the patient is close to dry weight.
Echocardiography provides relatively accurate measures of LV mass, cavity size, geometry, systolic function, and diastolic function. Systolic dysfunction is defined as an ejection fraction of less than 40%. It is often associated with LV dilatation (LV end-diastolic diameter greater than 5.6 cm), defined as LV cavity volume index of greater than 90 mL/m2 on echocardiogram (18). Concentric LVH is characterized by a thickened LV wall (greater than 1.2 cm during diastole) with normal cavity volume. LVMI is a calculated parameter that reflects the degree of hypertrophy. In non-CKD patients, the upper limits of normal are LVMI 130 g/m2 for men and 102 g/m2 for women (21). Although indexing LV mass to patient weight is the standard approach, it has been reported that LV mass indexed to patient height is more predictive of CV mortality in dialysis patients (22).
Cardiac magnetic resonance (CMR) imaging offers an alternative to echocardiography. It has been established as the most accurate noninvasive method of assessing ventricular dimensions in patients and is a useful tool in assessing cardiomyopathy in patients with ESKD (23–25). Compared to echocardiography, CMR has been shown to provide a more volume-independent measurement of the cardiac structure (26). In addition, echocardiography significantly overestimates LV mass relative to CMR in the presence of LVH and dilation, and this error is amplified in dialysis patients (27).
Other imaging techniques, such as nuclear medicine imaging of the LV using technetium-labeled red blood cells may be particularly useful in diagnosing areas of focal hypokinesis (usually resulting from ischemia). This method allows a more accurate estimate of global ejection fraction than does echocardiography alone.
Approximately half of patients presenting with a new diagnosis of HF have preserved ejection fraction (28). These patients have diastolic HF, and numerous society HF guidelines have consensus on the diagnostic criteria (28–32). The general criteria accepted by these guidelines include (a) clinical signs and symptoms typical of HF; (b) normal or only mildly reduced ejection fraction normal LV size and dimensions; (c) relevant structural heart disease including LVH, LA enlargement, and/or echo Doppler or catheterization evidence of diastolic dysfunction; and (d) a nonmyocardial cause of HF must be excluded. Overall, noninvasive measurements of diastolic function have low sensitivity, specificity, and predictive accuracy (33). The gold standard for diagnosis remains cardiac catheterization, which will directly demonstrate high LV filling pressures in the presence of normal ventricular volumes and contractility in these patients. Despite the limitations of echocardiography, however, it remains clinically useful and sufficient for diagnosis in most cases. An ejection fraction of greater than or equal to 50% is a more appropriate cutoff value to distinguish systolic from diastolic HF (34).
Because most dialysis patients have echocardiographic abnormalities at the beginning of ESKD treatment, it is a reasonable practice to perform M-mode and two-dimensional echocardiography on all patients at or before the start of ESKD therapy. This will provide baseline information for future comparisons, and the detection of LV disease at an earlier stage may allow more specific therapy to be employed in affected patients. Echocardiograms have the additional benefit of allowing detection of valve disease or a pericardial effusion, conditions that can potentially precipitate HF. Additionally, physicians may choose to give patients with significant myocardial dysfunction a somewhat different dialysis prescription than might otherwise be recommended. More frequent treatments or longer dialysis sessions may help the patient avoid symptomatic HF episodes. The Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines for the management of CV disease in patients with CKD recommend repeating the echocardiogram when there is a change in the patient’s clinical status and at 3-year intervals (35).
Outcome of Left Ventricular Disorders and Heart Failure
The presence of concentric LVH, LV dilatation with normal contractility, and systolic dysfunction at baseline has been associated with progressively worse survival, independent of age, gender, diabetes, and IHD (36). All three abnormalities are also associated with increased risk for the development of HF (FIGURES 16.2 and 16.3). This relationship between LV mass and CV events in dialysis patients has been confirmed, as was the prognostic impact of the different types of hypertrophy (22,37).
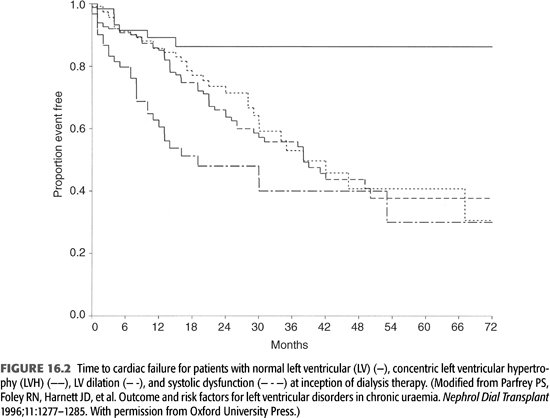
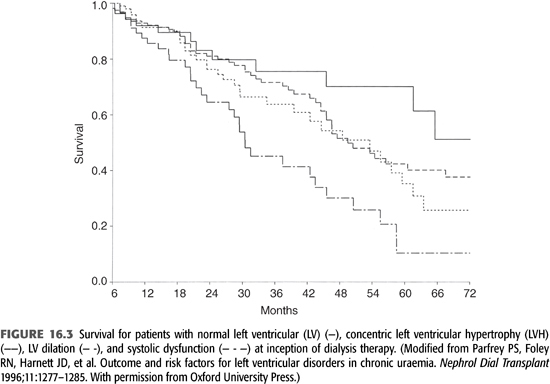
Symptomatic HF confers a poor prognosis for dialysis patients. In one cohort, the median survival of patients who had HF at or before initiation of ESKD therapy was 36 months compared with 62 months in subjects without baseline HF. This adverse prognosis was independent of age, diabetes, and IHD. Among patients who had HF at baseline, 56% developed recurrent HF and 44% remained failure-free during follow-up. Median survival in those with recurrent HF was 29 months, significantly less than in those without recurrence (45 months) (38). An analysis from the USRDS registry indicates that 1-year survival after treatment for HF is 68% for incident hemodialysis patients (2). It is interesting to note that HF has been consistently shown to be a strong independent risk factor for death, whereas the presence of IHD is not a significant risk factor for death independent of age, diabetes, and presence of HF (38). This suggests that the adverse impact of IHD exerts its effect through compromising LV pump function.
Risk Factors for Left Ventricular Disorders and Heart Failure
Independent predictors of HF at or before the time of ESKD therapy initiation include systolic dysfunction, older age, diabetes mellitus, and IHD. For patients without HF at baseline, predictors of the development of de novo HF include older age, systolic dysfunction, anemia, hypoalbuminemia, hypertension, and LVH (5,38). Numerous other risk factors, some unique to the dialysis patient, are known or suspected contributors to LV disorders. These include AV fistulas, disorders of divalent ion metabolism, chronic salt and water overload, altered oxidative stress, and chronic inflammation. The relative contribution of each of these risk factors in the pathogenesis of LVH has not been fully elucidated.
Nonmodifiable Risk Factor
Diabetes Mellitus
Diabetic patients without ESKD appear to be subject to a specific type of cardiomyopathy (39,40). Glycation of collagen may induce cross-linking of collagen fibers (41), and this may be a reason for large and small vessel diseases as well as myocardial dysfunction. In addition, LVH is more prevalent in hypertensive diabetic patients than in hypertensive nondiabetic patients (40,42).
In both the general and the dialysis population, diabetes is an independent risk factor for the development of HF and CAD (17,43,44). More than 50% of prevalent hemodialysis patients with a diagnosis of HF have diabetes (2). Some diabetic patients with ESKD have impairment of LV function despite normal coronary arteries, possibly resulting from the development of a diabetic cardiomyopathy as discussed earlier. Having said this, any analysis of the contribution of diabetes to the development of LV dysfunction in uremic patients is confounded by the very high prevalence of other risk factors, particularly hypertension.
The impact of diabetes was examined in a cohort study of dialysis patients who survived at least 6 months following the initiation of dialysis; 15% of these patients had insulin-dependent diabetes and 12% had non–insulin-dependent diabetes. On starting dialysis therapy, the prevalence of clinical manifestations of cardiac disease was significantly higher in diabetic patients compared with nondiabetic patients. Only 11% of diabetic patients had normal echocardiographic dimensions compared with 25% of nondiabetic patients, predominantly because of the prevalence of severe LVH (34% vs. 18%) (45). Older age, LVH, history of smoking, IHD, cardiac failure, and hypoalbuminemia were independently associated with mortality. Diabetes was a strong risk factor for the development of IHD but not for cardiac failure (45), suggesting that the excessive cardiac morbidity and mortality of diabetic patients may be mediated through ischemic disease rather than progression of cardiomyopathy while patients are on dialysis. Echocardiographic determination of LV size and function was a good predictor of survival. Diabetic patients receiving dialysis therapy with abnormal LV wall motion and abnormal LV internal diameter had the lowest mean survival (8 months), a mortality rate not matched by any subgroup defined by coronary anatomy, ventricular function, or clinical manifestation (46).
Ischemic Heart Disease
The risk factors and outcome for IHD are discussed elsewhere in this book. CAD is an important cause of systolic and diastolic dysfunction in the general population and in dialysis patients (17,47). Dialysis patients diagnosed with impaired LV systolic function should be evaluated for CAD.
Modifiable Risk Factors
Hypertension
The prevalence of hypertension is approximately 80% in hemodialysis patients, but it is closer to 50% in peritoneal dialysis patients (48). A cohort study of more than 2,500 adult hemodialysis patients reported that 86% had a systolic blood pressure (BP) greater than 150 mm Hg or a diastolic BP greater than 85 mm Hg; hypertension was controlled in only 30% of patients, and 12% were untreated (49).
The impact of hypertension was assessed in a cohort of 261 hemodialysis and 171 peritoneal dialysis patients. These patients were followed up prospectively for an average of 41 months per patient, and echocardiographic assessments were performed annually. The mean arterial BP level during dialysis therapy was 101 ± 11 mm Hg. When adjustments were made for age, diabetes, and IHD and hemoglobin (Hb) and serum albumin levels were measured serially, each 10 mm Hg rise in mean arterial BP was independently associated with the development of concentric LVH [odds ratio (OR) 1.48, p = 0.02], IHD (OR 1.39, p = 0.05), and de novo HF (OR 1.44, p = 0.007) (50). Hypertension was also associated with progressive concentric LVH in 596 incident hemodialysis patients without symptomatic cardiac disease or dilatation in a blinded, randomized controlled trial (51). Patients had echocardiograms within 18 months of starting dialysis and subsequently at 24, 48, and 96 weeks later. LVMI rose significantly during the study period from 114.2 g/m2 at baseline to 128.2 kg/m2 at week 96. High systolic BP was also associated with an increase in the composite outcome of CV events and death.
In the non–kidney disease population, reductions in BP are associated with regression of LVH. Although a similar effect in patients with ESKD seems highly likely, there is considerably less direct evidence that this is the case. In an attempt to determine the effect of BP lowering on LV size, while partially correcting anemia with erythropoietin, London et al. (52) enrolled 153 hemodialysis patients in a longitudinal study and followed them up for a mean of 54 months. The first step to control BP was achievement of dry weight. If this failed to achieve the target BP, an angiotensin-converting enzyme (ACE) inhibitor, calcium channel blocker, and then α-blocker was added as required. Predialysis BP decreased from 169/90 to 147/78 mm Hg, and Hb increased from 8.7 to 10.5 g/L. LVMI decreased from 174 to 162 g/m2. The hazard ratio associated with a 10% LV mass decrease was 0.78 for all-cause mortality and 0.72 for CV mortality. The authors concluded that alteration of hemodynamic overload favorably influenced the natural history of LVH in hemodialysis by reducing LVH, an outcome that had a beneficial effect on survival.
Current guidelines for the treatment of hypertension in the general population do not recommend different target BP for patients with and without LVH (53,54). The optimal target BP for patients requiring dialysis is not at all clear. In this group, low BP has been repeatedly associated with increased mortality (55,56). It is likely that low BP is a marker for the presence of cardiac failure (and/or other comorbidity), confounding analyses of BP and death. This hypothesis is supported by an analysis in the general population (57). In the absence of specific trial data, a conservative target is a predialysis BP of 140/90 mm Hg or less, unless the patient develops symptomatic hypotension during or after dialysis. This should be achieved primarily by maintenance of an accurate dry weight, with antihypertensive therapy added if satisfactory results are not achieved.
Anemia
There is considerable epidemiologic evidence that persistent anemia in patients with CKD is a risk factor for cardiac disease; it has been associated with LV dilatation and LVH in both CKD and ESKD patients [relative risk (RR) for LVH progression, per 10 g/L drop, is 1.74 in CKD and 1.48 in dialysis] (17,54–58). It is also a risk factor for the development of de novo HF and death but is not associated with de novo IHD (58).
Recombinant human erythropoietin is indicated for the treatment of anemia in patients with ESKD. The optimal treatment target for Hb, however, is subject to debate. Recent 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend starting erythropoietin-stimulating agents (ESAs) when the Hb is between 9 and 10 g/dL to avoid the Hb falling below 9 g/dL. ESAs should not be used to maintain Hb concentration of Hb above 11.5 g/dL as multiple randomized controlled trials have suggested that higher Hb targets may be harmful (59). Potential harms include increased rates of mortality CV events (60,61) and cerebrovascular events (62).
Numerous uncontrolled studies in CKD and dialysis patients have assessed the treatment of anemia using ESAs with the effect on LVMI as an outcome. One hundred forty-six hemodialysis patients without symptomatic cardiac disease were randomized to normalization of Hb with erythropoietin or to partial correction of anemia. Two groups were studied, patients with preexisting LV dilatation and patients with concentric hypertrophy. In the former group, mean LV volume was high at baseline (approximately 120 mg/m2) and a substantial minority had systolic dysfunction. Normalization of Hb failed to induce regression of LV dilatation. In the latter group, normalization of Hb failed to induce regression of LVH but it did prevent progressive LV dilatation (63).
Another randomized double blind trial involved of 596 hemodialysis patients without symptomatic cardiac disease and LV dilatation. Patients were allocated to lower (9.5 to 11.5 g/dL) and higher (13.5 to 14.5 g/dL) Hb targets using epoetin alpha. Both groups had increases in LV volume index (7.6% and 8.3%) and LVMI (16.8% vs. 14.2%). Thus, the investigators concluded that neither partial nor complete normalization of Hb has a beneficial effect on cardiac structure (62).
In contrast to these results, two studies showed favorable reductions in LVMI with correction of anemia. One study involved 230 patients and corrected Hb to 13.5 g/L for women and 14.5 g/L for men. A significant reduction in LVMI of −28 g/m2 was observed (64). However, optimization of HF therapy during this study confounds the true effect of anemia correction alone. A lower Hb target of 10.5 g/dL was used in a study of 153 hemodialysis patients that received parallel treatment of hypertension and anemia. This study showed a regression in LVMI by 12 g/m2 (52).
Whether treatment of anemia at an earlier stage of renal disease (e.g., in CKD) may be beneficial is still unclear. Small, uncontrolled studies have suggested that correction of anemia was associated with reductions in LVH (65,66). This has been contradicted by a trial in which 155 patients with CKD were randomized to a target Hb of 120 to 130 g/L versus 90 to 100 g/L. At the end of follow-up (2 years or the initiation of dialysis), there was no significant difference in changes in LVMI between the groups (67).
In order to further determine the impact of erythropoietin therapy on changes in the LVMI among CKD and ESKD patients, a meta-analysis of 15 studies, including those mentioned in the preceding text, involving 1,731 patients was performed. This study provided higher quality evidence that correction of severe anemia (Hb <10 g/dL) to a target of Hb ≤12 g/dL with recombinant erythropoietin resulted in significant reductions in LVMI [−32.7 g/m2, 95% confidence interval (CI) −49.2 to 16.1, p <0.05] (68). This meta-analysis was limited, however, because the studies lacked control groups and the studies involved patients with both CKD and ESKD. Furthermore, patients in many of these studies may have benefited from parallel treatment of hypertension and HF.
Although some authors have advocated individualized targets for patients with ESKD, there has yet to be any outcome-based studies to demonstrate either the safety or efficacy of this approach. Currently, it is reasonable to follow the most recent KDIGO guidelines for treatment of anemia for all patients including those with cardiac disease (59). These recommendations have been made based on studies demonstrating improvement in quality of life and exercise tolerance at Hb levels of 11.0 to 12.0 g/dL. At the same time, the current body of evidence provides no evidence of mortality benefit and a suggestion of potential for harm when anemia is fully corrected.
Hypoalbuminemia
Low albumin is a powerful predictor of poor outcome in dialysis patients and has been associated with LV dilatation, de novo HF, and IHD (69). The mechanisms underlying this association are unclear. It may be a marker for malnutrition, inadequate dialysis, vitamin deficiency, or a chronic inflammatory state. To date, there is no real evidence as to the impact of the correction of any these factors on cardiac function.
Volume Overload
Sodium and water overload causes plasma volume expansion. Blood volume correlates directly with LV diameter in hemodialysis patients, as does the magnitude of weight changes between sessions (70,71). Despite these associations, it is difficult to clearly discern cause and effect. It is possible that salt and water retention is induced by preexisting systolic or diastolic dysfunction in some patients rather than predisposing to it.
Keeping the patient’s dry weight optimal may minimize the degree of enlargement of the LV. It is interesting to note that LVH is more severe in long-term continuous ambulatory peritoneal dialysis (CAPD) patients than in hemodialysis patients (72). This finding is associated with evidence of more pronounced volume expansion, hypertension, and hypoalbuminemia.
In a retrospective study of 41 patients with symptomatic HF, the initiation of hemodialysis was associated with a significant decrease in LVMI by −24.3 ± 35.4 g/m2 (p <0.001) after 9 months of treatment. The authors of this study concluded the decrease in LVMI could not be explained by a decrease in BP but by better volume control and relief of venous congestion (73).
Abnormal Calcium and Phosphate Metabolism
There is considerable experimental and clinical evidence that the hyperparathyroid state associated with uremia contributes to cardiomyopathy, LVH, LV fibrosis, atherosclerosis, myocardial ischemia, and vascular and cardiac calcification (74–76). Registry data indicate that hyperphosphatemia and raised calcium × phosphate product are independent predictors of mortality (77), especially death from CAD and sudden death (78).
The appropriate use of dietary modification, vitamin D analogs, and phosphate binders are recommended to achieve target levels for serum calcium of 8.4 to 9.5 mg/dL, for serum phosphorus of 2.7 to 4.6 mg/dL, for calcium × phosphate product of 55 mg/dL, and for intact parathyroid hormone (PTH) 150 to 300 pg/mL (79). The availability of aluminum- and calcium-free phosphorus binders has significantly changed the management of ESKD patients. Sevelamer hydrochloride is an effective, although costly, phosphorus binder that is not associated with hypercalcemia. Meta-analysis suggests a survival benefit for non–calcium-based phosphate binders compared to calcium-based binders, but the evidence is weak (80). Evidence from the randomized trial of cinacalcet versus placebo in hemodialysis patients with moderate to severe hyperparathyroidism reveals a clinical benefit in the prevention of CV events in patients aged ≥65 years or in the prevention of severe unremitting hyperparathyroidism in all patients (81,82).
The recent Paricalcitol Capsule Benefits in Renal Failure-Induced Cardiac Morbidity (PRIMO) trial was a randomized, placebo-controlled trial involving 227 patients who assessed the effects of activated vitamin D on LV mass over 48 weeks (83). Enrolled patients had stage 3 and 4 CKD along with mild to moderate LVH. Despite sufficient suppression of immunoreactive parathyroid hormone (iPTH), paricalcitol failed to reduce LVMI over a 48-week period.
Valve Disease
Acquired aortic stenosis may occur in a few patients and may induce concentric LVH (84). Calcification of the aortic valve has been observed in 28% to 55% of dialysis patients in various series, whereas hemodynamically important stenosis has been reported in 3% to 13%. Progression at times may be extremely rapid. The major factors predisposing to aortic valve calcification appear to be hyperparathyroidism, duration of dialysis, and degree of elevation of calcium × phosphate product (85).
In ESKD patients, intimal arterial disease, age, duration of dialysis, and inflammation all appear to be predictors for valve calcification. The prominent risk factor for mitral annular calcification was diabetes (86).
Mode and Quantity of End-Stage Kidney Disease Therapy
The question as to whether higher dosing targets for dialysis than those currently recommended will result in improvements in cardiac outcomes has been addressed in two randomized controlled trials, with conflicting results. The recently published Frequent Hemodialysis Network (FHN) daily trial was a multicenter, prospective, randomized parallel group trial that compared frequent, six times per week, in-center hemodialysis to conventional three times weekly dialysis (87). The two coprimary outcomes were death or change in LV mass, as assessed by CMR imaging. Two hundred forty-five patients were randomized, and frequent dialysis was associated with significant benefits in the coprimary outcome (HR 0.61, 95% CI 0.46 to 0.82). Stand-alone reduction in LV mass was assessed as a secondary outcome, and the adjusted mean LV mass decreased by 16.4 ± 2.9 g in the frequent hemodialysis group, as compared with 2.6 ± 3.2 g in the conventional group. By comparison, in the Hemodialysis (HEMO) study, 1,846 patients on thrice-weekly hemodialysis were randomized to either “standard” dose (target equilibrated Kt/V = 1.05) or “high” dose (target equilibrated Kt/V = 1.45). No difference in the primary outcome of all-cause mortality or any of the prespecified secondary outcomes was observed between the groups (88). Chertow et al. (87) note that the benefit observed in the FHN trial may have been due to an even greater between-group difference in urea clearance compared to the HEMO study.
A recent randomized, controlled, open-label, blinded endpoint study aimed to determine if cooled dialysate provided long-term cardiac protection using serial CMR imaging (89). This study group had previously demonstrated that cooled dialysate can reduce recurrent myocardial stunning during HD. Seventy-three patients were initially randomized to either a dialysate temperature of 37°C or individualized cooling at 0.5°C below body temperature for 12 months. The investigators found no difference in the primary outcome of ejection fraction. However, there was a significant reduction in LV mass in the intervention group (decreased by 15.6 g compared to control) and a significant reduction of −23.8 mL in LV-end diastolic volume in the intervention group. Furthermore, global LV systolic function was maintained in the experimental group but decreased in the control group (difference of −3.3%, 95% CI −6.5% to −0.2%). The investigators concluded that individualized cooled dialysate slowed the progression of hemodialysis-associated cardiomyopathy. The generalization of these findings is limited as only 44 participants completed the study and the experimental group had a larger baseline LV mass (157.9 ± 50.5 g vs. 140.3 ± 48.7 g).
Although some patients who are unable to tolerate the intradialytic volume expansion associated with intermittent hemodialysis may be more easily managed with peritoneal dialysis, there is no good evidence that either modality is associated with improved outcomes for patients with heart disease. Nocturnal hemodialysis has been associated with an improvement in many clinical parameters, including BP and regression of LVH (90,91). Whether the observed cardiac benefits are due to amelioration of hypertension, improvement in anemia, or higher dialysis dose is not clear.
Kidney transplantation is undoubtedly the best treatment for ESKD, and a good model of what happens to cardiac function when uremia is optimally treated. Following kidney transplantation, concentric LVH and LV dilatation improves, but the most striking observation is the improvement in systolic dysfunction (92). It is not known which adverse risk factors characteristic of the uremic state have been corrected to produce the improvement in LV contractility, but hypertension and AV fistulas usually persist posttransplantation.
Management of Left Ventricular Disorders and Heart Failure
Asymptomatic LV disease is usually detected with screening echocardiography. Aggressive treatment of risk factors, particularly hypertension, anemia, and IHD, is critical to prevent further myocardial dysfunction. Periodic echocardiography to assess the efficacy of such measures is prudent, and careful observation for clinical evidence of HF is required.
The initial step in the treatment of any patient with symptoms of HF should be a careful assessment for reversible precipitating or aggravating factors. Arrhythmias, uncontrolled hypertension, and use of drugs that may adversely affect cardiac performance (e.g., most calcium channel blockers, most antiarrhythmic agents, or nonsteroidal anti-inflammatory drugs) are examples. IHD may be associated with HF. This diagnosis is not always obvious, especially in diabetic patients who may not have typical symptoms.
For some patients with CKD, the appearance of HF symptoms refractory to standard treatment heralds the need for dialysis initiation. ESKD patients with severe HF will usually require hemofiltration for relief of their acute symptoms, and those on maintenance dialysis will require careful assessment of their target weights. The maintenance of a patient’s true dry weight is of paramount importance in managing HF symptoms but may not be easily done in some cases. The occurrence of hypotension during dialysis, large interdialytic weight gains, and the possible overprescription of hemodynamically active drugs may need to be addressed. In difficult cases, ultrafiltration with simultaneous direct pressure monitoring using right heart catheterization may be helpful to define the optimal intravascular volume. HF unresponsive to changes in dry weight may be a complication of unsuspected IHD or valvular disease and should prompt further investigation.
Distinguishing systolic from diastolic dysfunction on clinical grounds is difficult, although the presence of hypertension with signs of HF is suggestive of hypertrophic disease with diastolic dysfunction. Systolic and diastolic dysfunction may coexist, and the relative contribution of each process may change with the evolution of LV disease in a given patient. Nonetheless, the clinical management of HF differs according to whether systolic or diastolic dysfunction predominates. An echocardiographic diagnosis of mainly diastolic disease could lead to changes in therapy, as discussed later. Consequently, an echocardiogram is an integral part of the evaluation of patients with HF.
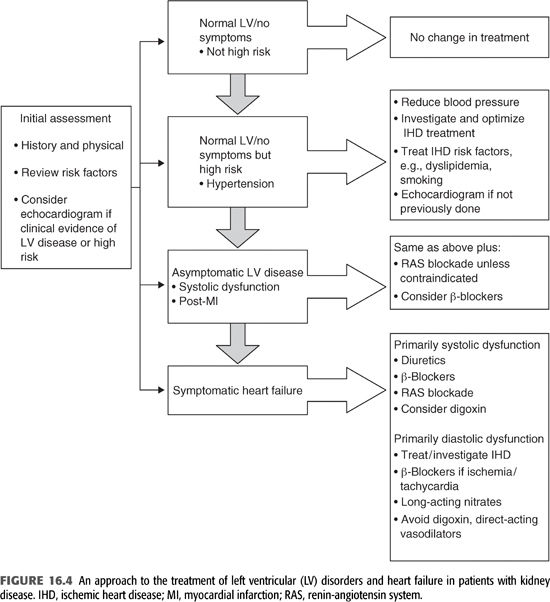
A suggested approach to the treatment of LV disorders and HF is shown in FIGURE 16.4.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


