PROTEIN-ENERGY WASTING IN MAINTENANCE DIALYSIS PATIENTS
Causes and Treatment of Protein-Energy Wasting
Protein-energy wasting (PEW) refers to the loss or reduced amounts of protein, fat, and/or carbohydrates in the body to an unhealthy degree. The term PEW rather than protein-energy malnutrition (PEM) is used because there are many causes of PEW which are not related to suboptimal nutrient intake. The many causes for PEW in maintenance dialysis (MD) patients are listed in TABLE 27.1 (1).

Reduced nutrient intake and nutrient losses into dialysate are important causes of PEW. Many studies indicate that dietary protein and energy intake are frequently low in MD patients (1,2). Dietary protein and energy intake often average 20% and 30%, respectively, below recommended intakes for maintenance hemodialysis (MHD) and chronic peritoneal dialysis (CPD) patients (1,2). Reports from uncontrolled studies of patients undergoing MHD at more frequent intervals (e.g., daily hemodialysis) and/or for more hours than the usual 3 to 4 hours of hemodialysis three times weekly indicate that their appetite and nutritional status improve (3). These findings suggest that MHD patients treated with standard dialysis therapy tend to remain somewhat anorexic, possibly because their uremia is less adequately treated. Hemodialysis with high-flux membranes removes about 8.0 ± 2.8 (SD) g and 9.3 ± 2.7 g of amino acids from postabsorptive and postprandial patients, respectively (4,5). Approximately 2 to 3.5 g/d of free amino acids are removed during continuous ambulatory peritoneal dialysis (CAPD) (6). Normally, little protein, perhaps 1 or 2 g of protein, is lost during hemodialysis. About 8.8 ± 0.5 standard error of the mean (SEM) g/d of total protein and 5.7 ± 0.4 g/d of albumin are lost into the dialysate with CAPD (7). With mild peritonitis, the quantity of protein removed increases to an average of 15.1 ± 3.6 g/d (7); protein losses can rise markedly with severe peritonitis. Such losses fall rapidly with antibiotic therapy but may remain elevated for many days to weeks, particularly if the peritoneal infection becomes well established before it is eradicated (7).
In normoglycemic individuals, approximately 15 to 25 g of glucose may be removed during hemodialysis when glucose-free dialysate is used (8). When the hemodialysate contains 200 mg/dL of glucose (180 mg/dL of anhydrous glucose), there is net absorption of approximately 10 to 12 g of glucose with each dialysis. Water-soluble vitamins and other bioactive compounds are removed by both hemodialysis and peritoneal dialysis (PD) (4–6,9). These vitamin losses can be easily replaced from the diet, but in patients with poor nutrient intake, such losses may enhance vitamin malnutrition. Patients with kidney disease often lose substantial quantities of blood secondary to occult gastrointestinal bleeding, frequent blood sampling for laboratory testing, and the sequestration of blood in the hemodialyzer (10). Since blood is rich in protein, these blood losses may contribute to protein wasting. The problem of maintaining adequate protein nutrition is potentially more difficult for CPD patients because the combined losses of proteins, peptides, and amino acids are greater than they are in patients undergoing hemodialysis, particularly since the losses with CPD occur every day, whereas hemodialysis is usually performed only three times weekly. Although the nutritional disorders most commonly associated with chronic kidney disease (CKD) are PEM and iron and vitamin D (e.g., 25-hydroxycholecalciferol and 1,25-dihydroxycholecalciferol) deficiencies (1,2,10–12), other deficiencies, particularly for vitamins B6 and C, folic acid, and possibly carnitine and zinc, occur frequently if the patient does not receive supplemental nutrients (9,13,14).
It is apparent from TABLE 27.1 that some of the causes of PEW in MD patients are not nutrition related. Other etiologic factors engendering PEW include inflammatory processes, the altered hormonal milieu of the patient with kidney disease, and both oxidant and carbonyl stress (15). Acidemia and physical deconditioning, which may be profound, may contribute to PEW. Because of the multifactorial nature of the wasting syndrome in patients with CKD, the term PEW (1) has been proposed as a more appropriate appellation for the wasting syndrome that occurs in CKD (16).
The multiple causes of PEW in MD patients require that therapeutic strategies to prevent and treat this condition should be directed into several areas. Some of these are indicated in TABLE 27.2.

Therapeutic strategies should be directed toward inaugurating MD in a timely manner, before patients with end-stage kidney failure develop frank PEW. PEW tends to occur as patients approach end-stage kidney failure (17,18) and inauguration of chronic dialysis treatment is associated with an improvement in PEW (19). In this regard, chronic dialysis patients are frequently anorectic (20). Therapeutic strategies for reduced nutritional intake include dietary counseling, food supplementation, intradialytic parenteral nutrition (IDPN), gastric or intestinal tube feeding, nutritional PD, or nutritional hemodialysis. In exceptional cases, total parenteral nutrition, given on a continuing basis, may be necessary (15).
Since MD patients frequently sustain intercurrent catabolic illnesses, it is important to treat such comorbid conditions aggressively and to ensure that there is adequate nutritional support during such treatment. MD patients are often in an inflammatory state and may have oxidant and/or carbonyl stress—even when there are no apparent superimposed complicating illnesses. It is believed that that elevated levels of inflammatory, oxidant, and carbonyl reactive compounds are important causes of morbidity and PEW in MD patients. CKD patients often have low circulating levels of certain anabolic hormones, increased levels of some catabolic hormones, and resistance to other anabolic hormones (15), and a number of studies have examined whether anabolic hormones may improve the protein-energy state of MD patients with PEW. Short-term studies in MHD or CPD patients given repetitive doses of growth hormone, insulin-like growth factor-1, or carnitine have shown anabolic effects of each of these agents (21–23).
Since acidemia can promote protein degradation (24), it is important to prevent and treat acidemia in these patients. In patients undergoing MHD thrice weekly, acidemia is particularly likely to occur immediately prior to a hemodialysis treatment, and it may be necessary to give such individuals alkali supplements during the end of their interdialytic period. MD patients with substantial residual renal function also may develop hyperchloremic acidosis due to excessive urinary bicarbonate losses. The optimal arterial blood pH for such patients has been reexamined in clinically stable patients undergoing CAPD. Metabolic balance studies in these patients indicate that arterial blood pH in the range of 7.43 to 7.45 is often associated with more positive protein balance than when their arterial blood pH is 7.36 to 7.38 (25).
Exercise training can improve both cardiopulmonary exercise capacity and strength in MD patients (26). Whether this treatment may be associated with protein accrual or increased muscle mass is not clear. We have found that exercise training in such patients is associated in skeletal muscle with increases in the gene transcripts for a number of growth factors and a reduction in the gene transcripts for myostatin, an antihypertrophic protein (27). However, these changes were not associated with an increase in skeletal muscle mass possibly because patients did not exercise sufficiently vigorously or that kidney failure is an antianabolic state. The increased gene transcripts may have induced protein remodeling that resulted in the increased exercise capacity that occurs in MD patients who exercise train (26). It should be emphasized that there are no randomized, prospective, controlled clinical trials that have examined whether improving the protein-energy state of wasted MD patients will reduce morbidity or mortality or improve quality of life. However, retrospective analyses of patients receiving intradialytic nutrition suggest that nutritional support in these patients is associated with reduced mortality (28–30), and exercise training, at least in the short term, may improved quality of life scores.
Effects of Protein-Energy Wasting on the Clinical Course of Maintenance Dialysis Patients
Many epidemiologic studies indicate that the PEW status of MHD and CPD patients is strongly associated with increased morbidity and mortality. MD patients who either have PEW or who have worsening protein-energy status have substantially higher mortality rates; the increase in mortality is largely due to cardiovascular events (20,31). MD patients who ingest inadequate quantities of protein and energy are more likely to develop PEW and to have higher mortality (20,32). Serum albumin is one of the strongest predictors of mortality in MHD patients (31,33,34). There is a direct relationship morbidity or mortality rates in MHD patients and poor appetite; spontaneous low dietary protein intake; decreased body-weight-for-height [i.e., body mass index (BMI)]; anthropometric measures of skeletal muscle mass and total body fat; and low serum albumin, urea, creatinine, cholesterol, and potassium (20,31–34). Similar relationships with morbidity and mortality, based on smaller sample sizes, usually (33,35), but not always (36), have been shown for CPD patients. The relative contributions of PEM and inflammation to the high morbidity and mortality of CKD patients are unclear, particularly because the syndrome of PEM shares many clinical manifestations with inflammation. Because inflammatory processes may cause endothelial injury and/or predispose to atherosclerosis and vascular thrombosis, it is easy to perceive why there could be a causal connection between inflammation and morbidity and mortality from vascular disease.
 DIETARY THERAPY FOR MAINTENANCE DIALYSIS PATIENTS
DIETARY THERAPY FOR MAINTENANCE DIALYSIS PATIENTS
General Approach to Dietary Management
The widespread nutritional and metabolic alterations and high incidence of malnutrition in CKD patients indicate that nutritional therapy is a critical aspect of their management. The three main goals for dietary treatment are to maintain good nutritional status; to prevent or ameliorate uremic toxicity and the metabolic disorders of kidney disease; and to reduce the risks of cardiovascular, cerebrovascular, and peripheral vascular disease. Adherence to specialized diets is often a difficult and frustrating endeavor for patients and their families. Patients following low-protein diet usually must make fundamental changes in their behavior patterns and forsake some of their traditional sources of daily pleasure. Often, the patients must procure special foods, prepare special recipes, forego or severely limit their intake of favorite foods, or eat foods that they may not desire. To ensure successful dietary therapy, patients with kidney disease must undergo extensive training concerning the principles of nutritional therapy and the design and preparation of diets, and they need to be repetitively encouraged to adhere to the prescribed diet. They usually require repeated training regarding their nutritional therapy. Without careful monitoring of nutritional intake, retraining, encouragement, and sensitivity to their cultural background, psychosocial condition, and lifestyle, patients will be more likely to adhere poorly to their dietary prescription. They may eat too little rather than too much of some essential nutrients.
Monitoring Compliance
In general, to maintain good dietary adherence and to maintain a healthy clinical, fluid and electrolyte, and nutritional status, the physician must review the patient’s nutritional status and dietary compliance at frequent intervals. Since nutritional status may begin to deteriorate in patients with chronic progressive kidney disease when the glomerular filtration rate (GFR) falls below 35 to 50 mL/min/l.73m2 (17,18), careful attention to nutritional status should begin at this time or earlier. This is particularly important because CKD patients appear to be at greatest risk for malnutrition from the time that the GFR falls below 10 mL/min until the patient is established on MD therapy (18,37). Also, although nutritional status often improves after commencing hemodialysis (19), nutritional status of patients at the onset of chronic dialysis treatment is a good predictor of their nutritional status 2 to 3 years later (37,38). Moreover, measures indicating PEW at the onset of MD therapy predict increased morbidity and mortality (33,39). Hence, particular effort should be given to prevent malnutrition as the patient approaches dialysis therapy and during the first few weeks of MD treatment. These efforts should be directed toward maintaining good nutritional intake during this period, rapidly instituting therapy for superimposed illnesses, and maintaining good nutritional support during such illnesses.
Monitoring Nutritional-Inflammatory Status
Since diets prescribed for renal insufficiency are often marginally low in some nutrients (e.g., protein) and high in others (e.g., calcium) and PEW is not infrequent, it is important to periodically evaluate the adequacy of the patient’s diet and protein-energy status. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF K/DOQI) Clinical Practice Guidelines for Nutrition in Chronic Renal Failure (40) recommends that panels of nutritional measures should be used to assess protein-energy nutritional status in MD patients. The recommended panel of measurements of PEW status for MHD and CPD patients is indicated in TABLE 27.3. A predialysis serum specimen for these measurements is obtained from an individual immediately before the initiation of a chronic hemodialysis or intermittent peritoneal dialysis (IPD) treatment. A stabilized serum measurement is obtained after the patient has stabilized on a given dose of CAPD or IPD. There are other nutrition-related parameters that often will require monitoring, for example, serum albumin, potassium, phosphorus, calcium, parathyroid hormone, iron, ferritin, transferrin, total low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol and triglycerides, interdialytic weight gain, and, if relevant, bone densitometry or radiography (15). Measures of inflammation or oxidant or carbonyl stress are often very helpful. Such measures usually include serum quantitative high sensitivity C-reactive protein (CRP) and serum interleukin-6 (15). Dietitians are often best qualified to perform anthropometric measurements of nutritional status because of training and experience.
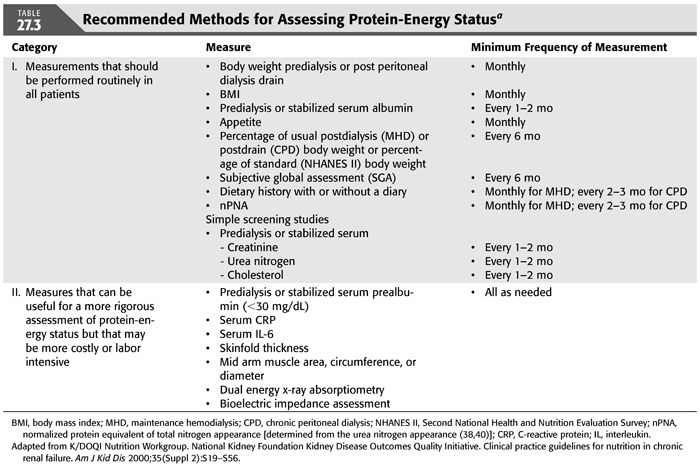
 RECOMMENDED DIETARY INTAKES
RECOMMENDED DIETARY INTAKES
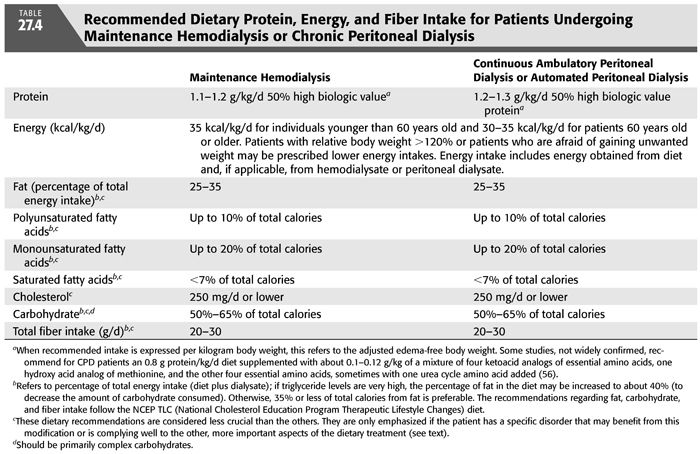
The nutritional needs of patients treated with more frequent hemodialysis have not been systematically investigated. Uncontrolled reports indicate that patients treated with more frequent hemodialysis have greater appetites and food intake (3). It is anticipated that such individuals will probably have increased daily needs for certain nutrients than are discussed in this chapter and listed in TABLE 27.4. Such patients may also have greater tolerance for some nutrients than is indicated here. The benefits of more frequent hemodialysis have not yet been established in randomized, prospective clinical trials, although less well controlled data are very encouraging (41,42).
Protein
Maintenance Hemodialysis
Protein requirements are increased in MHD patients because of the removal of amino acids and peptides by the dialysis procedure (4,5) and because the hemodialysis treatment appears to stimulate protein catabolism by engendering an inflammatory, catabolic response (43). Nitrogen balance studies suggest that many MHD patients may require at least 1.0 g protein/kg/d to maintain both protein balance and normal total body protein mass (44). However, for a safe protein intake that will maintain protein balance in almost all clinically stable MHD patients, the NKF K/DOQI Clinical Practice Guidelines on Nutrition in CKD recommend 1.2 g protein per kilogram body weight per day (40) and the European Best Practice Guidelines (EBPG) recommend at least 1.1 g protein per kilogram ideal body weight (IBW) per day (45). To ensure adequate intake of essential amino acids, it is recommended that at least half of the dietary protein should be of high biologic value, although experimental evidence to prove this contention is lacking. For most individuals undergoing more frequent MHD, 1.2 g protein/kg/d probably will also provide an adequate protein intake.
Chronic Peritoneal Dialysis
A diet providing 1.2 to 1.3 g protein/kg/d appears to be a safe protein intake for CAPD patients and is consistent with the recommendations of the NKF K/DOQI Clinical Practice Guidelines on Nutrition in CKD (40) and with complete nitrogen balance studies on CAPD patients (46). Daily losses of amino acids and protein into dialysate of patients undergoing automated daily peritoneal dialysis (APD) are similar to the losses with CAPD (6,7,47). Thus, although there are no complete nitrogen balance studies of dietary protein requirements in APD patients, one would expect that the dietary protein requirements would be similar to those of patients undergoing CAPD. Again, it is recommended that at least 50% of the dietary protein should be of high biologic value (40). It is possible that CAPD and APD patients who are protein depleted or hypercatabolic may become more anabolic when they ingest up to 1.5 g protein/kg/d, although this has not been clinically investigated.
Patients classified as high peritoneal transporters by the peritoneal equilibration test lose more protein and amino acids into peritoneal dialysate than those classified as low transporters (e.g., typical 24-hour losses in high vs. low transporters: albumin, 4.9 ± 2 (SD) vs. 3.2 ± 1 g/d, p <0.03, total free amino acids, 15.4 ± 4 vs. 10 ± 4 mmol/d, p = 0.002) (48,49). This trend is shown in all studies of high versus low transporters. The high transporters also have, on average, lower serum albumin levels (48,49). However, the difference in protein and amino acid losses with high versus low transporters is not great, and dietary protein intakes of 1.2 to 1.3 g/kg/d should provide sufficient amounts of protein and amino acids to compensate for these increased peritoneal losses if the ability to synthesize these serum proteins is normal.
Some nephrologists report MHD or CPD patients who habitually ingest somewhat lesser amounts of protein or calories than are recommended and yet do not show evidence of PEW and lead physically active, rehabilitated lives. These observations raise questions as to whether the foregoing recommended dietary protein and energy intakes for chronic dialysis patients may be excessive. Several comments may be pertinent in this regard. First, some MD patients who appear to be doing well will, on close inspection, turn out to have evidence for malnutrition. Epidemiologic studies have associated protein depletion, even in mild forms, with increased mortality (31–35,50). Second, subtle forms of malnutrition are particularly difficult to detect, and the recommended intakes may provide some protection against mild forms of PEM. Third, the concept of dietary allowances presupposes that in order to ensure a sufficient nutrient intake for virtually all individuals (i.e., about 97%) in a given population, the recommended allowance must be greater than the actual requirement for a large proportion of that population (51). This reasoning is similar to that used by the World Health Organization and the Food and Nutrition Board of the National Academy of Sciences to determine the recommended dietary protein intakes for normal adults (52). Thus, it is expected that some individuals will ingest less protein or calories than is recommended without developing protein malnutrition. At present, there is no method that will identify in advance, under the conditions of clinical practice, which patients can safely ingest lower protein or energy intakes. Hence, to be safe, unless the patient can be shown to maintain a healthy nutritional status on a lower nutrient intake, he or she should be prescribed the dietary allowances described here.
Recently, a number of studies have reported that patients with far advanced or end-stage CKD may be treated with low-protein or very low-protein diets (e.g., 0.60 g protein/kg/d or 0.3 to 0.4 g protein/kg/d) supplemented with ketoacid or hydroxyacid analogs of usually five essential amino acids plus the other four essential amino acids. These studies indicate that for many patients, this treatment may safely delay the need for chronic dialysis therapy (53), may safely allow time for a PD access or a hemodialysis vascular access to mature without, for example, resorting to tunnel catheters (54), or may allow for a lower dose of dialysis treatment, at least as long as some residual kidney function is preserved (55). Some data suggest that these low-protein or supplemented very low-protein diets may delay the loss of residual kidney function (55,56). These diets appear to attain these results without inducing protein or energy wasting, serious metabolic abnormalities, or uremic toxicity. Some data suggest that the ketoacid and essential amino acid preparations may induce an anti-inflammatory effect (57). For the low-protein diets or a combination of low-protein diets and dialysis treatments to be safe and effective for patients with advanced CKD or end-stage kidney disease (ESKD), it would seem that the patient must be very motivated to adhere to the protein-restricted diets.
Energy
In most studies, energy expenditure in patients undergoing MHD or CAPD, measured by indirect calorimetry, appears to be normal during resting and sitting, following ingestion of a standard meal, and with defined exercise (58). What is most impressive about these foregoing studies is that there is no report of decreased energy expenditure in CKD, MHD, or CPD patients. Dietary energy intake in MHD and CPD patients is commonly reported to be lower than their recommended intakes (1,2). Many CPD patients tend to gain body fat and weight, probably due to the glucose uptake from peritoneal dialysate. The surges in plasma insulin that accompany the glucose absorbed from peritoneal dialysate may contribute to the increase in body fat that is not uncommonly observed in CPD patients.
The NKF K/DOQI Clinical Practice Guidelines for Nutrition in CKD recommend an energy intake of 35 kcal/kg/d for MHD and CPD patients who are younger than 60 years of age and 30 to 35 kcal/kg/d for those who are 60 years of age or older (TABLE 27.4) (40). This intake includes energy derived from the diet as well as from any fuels taken up from dialysate. Because individuals older than 60 years old tend to be more sedentary and their muscles often constitute a smaller proportion of their body mass, their recommended energy intake is somewhat lower. These recommendations are similar to the EBPG of 30 to 40 kcal/kg IBW/d adjusted for age, gender, and estimated physical activity (45). These intakes are also rather similar to those for normal individuals engaged in light to moderate activity as put forth in the RDA by the Food and Nutrition Board, National Academy of Sciences (51). Patients who are obese with an edema-free body weight greater than 120% of desirable body weight may be treated with lower calorie intakes. Some patients, particularly young or middle-aged women, may become obese on this energy intake or may refuse to ingest the recommended calories out of fear of obesity. These individuals may require a lower energy prescription. There are many readily prepared or commercially available high-calorie foodstuffs that are low in protein, sodium, and potassium which can be recommended by the renal dietitian.
 TREATMENT OF RISK FACTORS FOR VASCULAR DISEASE
TREATMENT OF RISK FACTORS FOR VASCULAR DISEASE
Lipids
There are a number of reviews of the causes for the abnormal serum lipids and lipoproteins in MHD and CPD patients, and space constraints do not allow a full discussion of these abnormalities or their causes (59). In brief, MHD and CPD patients often have increased serum triglyceride levels, intermediate density lipoprotein (IDL), and very low-density lipoprotein (VLDL), and serum lipoprotein(a) [Lp(a)]; serum HDL cholesterol is often low. CPD patients often have higher serum total cholesterol, triglycerides, LDL cholesterol, and apolipoprotein B levels than do MHD patients. Qualitative changes in the apolipoprotein concentrations also occur; among these is an increase in small dense low-density lipoprotein (sd LDL) (60). Since alterations in lipid metabolism and serum lipids may contribute to the high incidence of atherosclerosis and cardiovascular cerebrovascular and peripheral vascular disease in CKD patients, attention has been directed toward reducing serum triglycerides and LDL cholesterol and increasing HDL cholesterol.
Treatment of altered lipid levels and the risk of cardiac and vascular disease normally involves three components: nutrient intake, medicines, and exercise. In MHD patients, clinical trials do not indicate that cholesterol-lowering agents reduce adverse cardiovascular events (59), and there are no clinical trials examining whether any dietary lipid therapy reduces cardiovascular risk. On the other hand, epidemiologic studies indicate that serum levels that are low in phosphorus and a lower serum calcium–phosphorus product are associated with less cardiovascular disease (61,62).
Until more evidence is available, based on data from the general population, we encourage MD patients to follow a dietary plan that is based upon the National Cholesterol Education Program (NCEP) Therapeutic Lifestyle Changes (TLC) diet for all MHD and CPD patients, especially if their serum LDL levels are over 100 mg/dL (63). Since these patients are at high risk for cardiovascular, cerebrovascular, and peripheral vascular disease, we prefer to set a target LDL cholesterol of 70 mg/dL. The TLC diet provides the following (63): no more than 25% to 35% of total calories from fat, polyunsaturated fatty acids providing up to 10% of total calories, monounsaturated fatty acids providing up to 20% of total calories, saturated fatty acids providing less than 7% of total calories, and a cholesterol content of 200 mg/d or lower. Carbohydrate intake should be 50% to 60% of total calories and derived predominantly from foods rich in complex carbohydrates. Fiber intake should be 20 to 30 g/d (63). Since the TLC diet may be less palatable to many patients, their energy intake should be monitored to ensure that it remains adequate (see the subsequent text). Patients are encouraged to control calorie intake to avoid becoming substantially overweight or frankly obese. (i.e., avoid a BMI greater than about 28 kg/m2). It is recognized that most individuals will not be able to adhere exactly to the TLC diet. However, there is reason to believe that even some modifications of dietary intake in the direction of lowering serum cholesterol may reduce the risk of adverse vascular events (64). Moreover, if MD patients are not counseled on the characteristics and rationale underlying this diet, they may eat even a less healthy diet as they grow older.
Omega-3 fatty acids (e.g., eicosapentaenoic acid and docosahexaenoic acid, which are found in fish oil) lower serum triglycerides and have more variable effects on serum LDL cholesterol and HDL cholesterol (65). Fish oil also decreases platelet aggregation and appears to exert anti-inflammatory effects, and omega-3 fatty acids may enhance immune function. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) lower LDL-cholesterol; may slightly increase serum HDL-cholesterol; appear to be anti-inflammatory, antithrombotic, and fibrinolytic; improve impaired endothelial function; and, in animals, protect against progressive renal injury (66). It is emphasized that evidence that manipulation of lipid intake may reduce cardiovascular risk in MHD and CPD patients is currently lacking.
Other Potential Nonnutritional Techniques for Reducing Cardiovascular Risk
Evidence for the protective nature of other methods for reducing cardiovascular risk is largely obtained from clinical trials in the general population. Antiplatelet therapy, such as aspirin, has been shown to reduce the risk of myocardial infarction in adult high-risk CKD patients (67,68). In CKD patients, impaired hemostasis caused by aspirin may increase the risk of bleeding. However, 100 mg aspirin per day taken by MD patients increased the risk of minor bleeding (e.g., epistaxis, ecchymoses, or bruising) by threefold (p = 0.001) but not the risk of major bleeding episodes (e.g., leading to hospitalization or fatality) (69).
Multifactorial intervention may reduce the risk of adverse cardiovascular events in patients with type 2 diabetes mellitus without advanced kidney failure (70). The intervention consisted of dietary treatment (fat intake less than 30% of daily energy intake, saturated fatty acids less than 10% of daily intake), 30 minutes of light to moderate exercise three to five times per week, smoking cessation classes for smokers, daily intake of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) irrespective of blood pressure, daily vitamin-mineral supplements providing vitamin C 250 mg, D-α-tocopherol 100 mg, folic acid 400 μg, chromium picolinate 100 μg, aspirin (unless there were contraindicators), and aggressive control of blood glucose, blood pressure, and hypercholesterolemia and hypertriglyceridemia. The patients receiving this intensive therapy had a significantly lower risk of adverse cardiovascular events, kidney disease (albuminuria greater than 300 mg/24 h in two of three consecutive urine specimens), retinopathy, and autonomic neuropathy. We treat hypertriglyceridemia by dietary modification or medicines only when fasting serum triglycerides are very high (i.e., 500 mg/dL or more), because in the general population, these triglyceride levels increase the risk of adverse cardiovascular events and pancreatitis. In this situation, dietary fat intake may be increased but not above 40% of total calories. A high proportion of dietary carbohydrates should be complex. These modifications often lower the palatability of the diet; therefore, the patient’s total energy intake must be monitored closely to ensure that it does not fall to levels that are inadequate to maintain desirable body and protein mass. With high serum triglyceride levels that are unresponsive to dietary therapy, a fibrate (e.g., fenofibrate) may be tried cautiously while monitoring the patient for myopathy.
Oxidant and Carbonyl Stress
As indicated earlier, end-stage kidney disease is associated with increased levels of compounds that promote oxidant and carbonyl stress and chronic inflammation, all of which may promote atherosclerotic and proliferative vascular disease (71,72). Although there are few interventional trials evaluating the effects of reduction of these risk factors on morbidity or mortality of MD patients, the following treatments may be considered for MHD patients, particularly given their high risk of cardiovascular disease: (a) larger flux dialyzers that remove greater amounts of advanced glycation end products and other reactive carbonyl compounds; (b) antioxidants or antioxidant precursors, such as vitamins E or C (see the section “Vitamins” in the subsequent text) or selenium; supplemental selenium must be taken with caution because selenium is primarily excreted in the urine and may accumulate in kidney disease (73); (c) one glass per day of an alcoholic drink, perhaps particularly red wine; (d) statins, notwithstanding the lack of evidence for their effectiveness in MHD patients; and (e) regular exercise.
Carnitine
Carnitine, which is essential for life (74), is both ingested and synthesized in vivo. Carnitine facilitates the transfer of long-chain (greater than 10 carbon) fatty acids into muscle mitochondria. Since fatty acids are the major fuel source for skeletal and myocardial muscle at rest and during mild to moderate exercise, carnitine is considered necessary for normal skeletal and cardiac muscle function. MD patients and particularly MHD patients display low serum-free carnitine and, in some but not all studies, low skeletal muscle-free and total carnitine levels (74). Also, in MHD patients, serum and sometimes muscle acylcarnitines (fatty acid-carnitine compounds) and serum total carnitines are increased (75). The low serum and skeletal muscle-free carnitine levels led some investigators to postulate that many MD patients are carnitine deficient. Clinical trials of oral or intravenous carnitine administration to MD patients have led to conflicting results. Most students of this problem believe that evidence for beneficial effects of carnitine supplements to MD patients is unconvincing.
Carbohydrates
Patients should be encouraged to eat complex rather than purified carbohydrates to reduce triglyceride synthesis and to improve glucose tolerance if it is abnormal.
Minerals and Vitamins
Sodium
Hypertension in MD patients generally is more easily controlled when they are sodium restricted, and hypertension may be accentuated by an increased sodium intake, probably because of expanded extracellular fluid volume (76) and possibly due to altered intracellular electrolyte composition within arteriolar smooth muscle cells that increase contractility. Cross-sectional studies indicate that the blood pressure that is associated with lowest mortality are increased in MHD patients. The range of prehemodialysis systolic and diastolic blood pressures associated with the lowest mortality in MHD patients is approximately 160 to 189 mm Hg and 70 to 99 mm Hg, respectively (77). However, longitudinal studies of MHD patients indicates blood pressures closer to healthy levels for the general population are associated with improved survival.
It is recommended that blood pressures for CPD patients and for MHD patients, obtained prehemodialysis, should be no higher than 140/90 mm Hg (78). If sodium and water balance are tightly controlled in MHD patients, most such individuals will require little or no antihypertensive medications to maintain a desirable blood pressure. However, this level of sodium control is difficult to attain with hemodialysis performed only thrice weekly for ≤4 hours per hemodialysis treatment. Usually, when sodium balance is well controlled, the thirst mechanism will adequately regulate water balance. In patients with diabetes mellitus, hyperglycemia may also increase thirst and enhance positive water balance. Some MD patients are at risk for overhydration from excessive water intake independent of sodium intake, and their water intake should be controlled independently of sodium. In patients undergoing MD who are not anuric and who gain excessive sodium or water despite attempts at dietary restriction, a potent loop diuretic, such as furosemide or bumetanide, may be tried to increase urinary sodium and water excretion.
Patients undergoing MHD or CPD frequently are oliguric or anuric. For MHD patients, sodium and total fluid intake generally should be restricted to 1,000 to 2,000 mg/d and 1,000 to 1,500 mL/d, respectively. Because sodium and water can be removed easily with CAPD or other forms of daily PD, a more liberal salt and water intake is usually allowed. Indeed, by maintaining a larger dietary sodium and water intake, the quantity of fluid removed from the CPD patient and hence the daily dialysate outflow volume can be increased. This may be advantageous, since with CPD, the daily clearance of small- and middle-sized molecules is directly related to the volume of dialysate outflow. Therefore, for some CPD patients, a higher sodium and water intake (e.g., 6 to 8 g/d of sodium and 3 L/d of water) may enable the patient to use more hypertonic or hyperoncotic dialysate to increase the dialysate outflow volume, thereby increasing dialysate clearances and energy uptake from dialysate, if hypertonic glucose is used. This treatment may be undesirable for obese or severely hypertriglyceridemic patients because the greater use of hypertonic glucose exchanges will increase their glucose load. Also, there is the potential disadvantage that some patients may become habituated to high salt and water intakes; if they change to hemodialysis therapy, they may have difficulty curtailing their sodium and water intake.
Potassium
MD patients exhibit increased fecal potassium (46), which slightly increases the tolerance to dietary potassium. Loss of renal function, acidemia, catabolic stress, hypoinsulinism or insulin resistance, and catecholamine antagonists may each be associated with increased risk of hyperkalemia in MD patients (79). Hyperkalemia can usually be prevented in MD patients if they ingest no more than 70 mEq of potassium per day. Refractory hyperkalemia may require treatment with lower dialysate potassium, oral intake of potassium binders, or more frequent hemodialysis.
Magnesium
The optimal dietary magnesium allowance for MD patients has not been well defined. Experience suggests that when the magnesium content is about 1.0 mEq/L in hemodialysate or 0.50 to 0.75 mEq/L in peritoneal dialysate, a dietary magnesium intake of 200 to 300 mg/d will maintain the serum magnesium at normal or only slightly elevated levels.
Phosphorus and Phosphate Binders
The rationale for controlling dietary phosphorus and methods to prevent and treat hyperphosphatemia, a high serum calcium–phosphorus product, calcium phosphate deposition in soft tissue, hyperparathyroidism, and altered bone mineral metabolism are discussed in Chapter 24. This section considers the prescription of dietary phosphorus intake and phosphate binders. In MD patients, a large dietary phosphorus intake can lead to a high plasma calcium–phosphorus product with increased risk of calcium and phosphate deposition in soft tissues including arteries. Moreover, hyperphosphatemia, by lowering serum calcium, provides a strong stimulus to the development of hyperparathyroidism and increased serum FGF-23 levels. Hyperphosphatemia, hyperparathyroidism, and increased FGF-23 are independently associated with increased mortality in MHD patients (33,80). The NKF KDOQI Clinical Practice Guidelines recommend for MHD and CPD patients that serum phosphorus be maintained between 3.5 and 5.5 mg/dL (11). This often requires a low phosphorus intake not to exceed 1,000 to 1,200 mg/d, (i.e., about 12 to 15 mg phosphorus/kg/d), particularly when MD patients have moderate or severe hyperparathyroidism (see the subsequent text). This higher upper limit was chosen because with their greater protein intakes, dialysis patients cannot readily ingest less phosphorus without making the diet too restrictive and unattractive. Without phosphate binders, there is a net intestinal phosphate absorption (diet minus fecal phosphorus) of roughly 60% of the phosphorus intake (46). Therefore, even with this level of dietary phosphorus restriction, MHD and CPD patients almost always require phosphate binders to prevent hyperphosphatemia, unless MHD patients undergo hemodialysis more than thrice weekly. Serum phosphorus levels should be monitored monthly after starting MD, and dietary phosphorus restriction should be employed with use of phosphate binders as necessary to ensure that serum phosphorus remains within the normal range.
The phosphate binders, aluminum carbonate and aluminum hydroxide, are uncommonly used now because of aluminum toxicity (81). Phosphate binders in current use include calcium carbonate, calcium acetate, sevelamer hydrochloride (82), sevelamer carbonate, lanthanum carbonate (83), and several iron preparations (84,85). These phosphate binders are discussed in greater detail in Chapter 24. Phosphate binders vary somewhat as to the effectiveness at binding phosphate in the intestinal tract and not uncommonly cause such mild gastrointestinal distress as anorexia, nausea, diarrhea, or constipation which may reduce compliance (86). Often, with more time taking the same binder, these symptoms will abate. Calcium binder doses should not provide more than about 600 to 1,500 mg of elemental calcium per day [total calcium intake (from diet plus binders) should not exceed 1,000 to 2,000 mg/d] to prevent excessive accumulation of calcium in soft tissues and especially arteries (11,13). Treatment with 1,25-dihydroxycholecalciferol (calcitriol) or its analogs may decrease tolerance to calcium binders by enhancing intestinal calcium absorption. Calcium binders should be taken in divided doses with meals and should not be prescribed if the serum phosphorus level is very high in order to avoid precipitation of calcium and phosphate in soft tissues. Therefore, hyperphosphatemic patients may be treated with other binders of phosphate until serum phosphorus falls to normal or near normal. At that time, the regimen may be changed to a calcium binder. Calcium comprises 40% of calcium carbonate and 25% of calcium acetate.
Noncalcium binders have the advantage that they should pose no increased risk of calcium deposition in soft tissues including arteries. Sevelamer HCl is often given in doses of two to six 800 mg capsules three or four times per day with meals. Sevelamer HCl also has the benefit of lowering serum LDL-cholesterol and increasing serum HDL-cholesterol (87). Some individuals taking this drug can become acidemic because of the large hydrochloride content of this preparation. Sevelamer carbonate, which should not induce acidemia, can be prescribed instead (88). Lanthanum carbonate appears to be one of the more effective phosphate binders and is generally well tolerated. Small quantities of lanthanum accumulate in tissues of MD patients with daily doses (89); at present, there is no evidence that this is harmful to patients. Lanthanum carbonate is often given in doses of 0.5 to 1.5 g three times daily with meals. Ferric citrate and sucroferric oxyhydroxide are currently available iron-based phosphate binders (85,90). These compounds may also provide an iron supplement and may decrease the amount of intravenous iron and the dosage of erythropoiesis-stimulating agents that MD patients may require (84,91). Bixalomer, a phosphate-binding polymer, may also be tried (92). If MD patients are intolerant to recommended doses of any of these binders or if they remain hyperphosphatemic despite maximal or maximally tolerated doses of a binder (serum phosphorus greater than greater than 5.5 mg/dL), two or more phosphate binders may be given simultaneously. Phosphate binders, in general, bind only up to 300 to 500 mg phosphorus per day, even when given in maximum doses. Since people absorb roughly 60% of ingested phosphorus, the use of phosphate binders does not substitute for the need to restrict dietary phosphorus unless patients are receiving quotidian dialysis (93).
Some studies indicate that compounds that impair phosphate transport in the intestinal tract may also decrease serum phosphorus and the body phosphate burden. This has been shown for niacin and tenapanor (94,95).
Calcium, Vitamin D, and Parathyroid Hormone
The NKF DOQI guidelines recommend the serum calcium–phosphorus product should be maintained below 55 mg2/dL2 (11). This product should be attained primarily by controlling serum levels of phosphorus within the target range as indicated earlier. Frequent monitoring of serum calcium is important because hypercalcemia may develop, particularly if serum phosphorus should fall to low-normal or low levels. This is especially likely to occur if the patient also has hyperparathyroidism, a common complication of CKF, or is receiving larger doses of calcitriol. The NKF DOQI guidelines recommend that serum calcium levels, corrected for any alteration in serum albumin (see the subsequent text), should be maintained within the normal range for the laboratory used but preferably toward the lower end of normal (8.4 to 9.5 mg/dL) (11). As indicated earlier, it is the authors’ policy that total calcium intake from the diet and calcium-based phosphate binders combined should not exceed 1,000 to 2,000 mg/d. If the serum total corrected calcium is below the lower limit for the laboratory used (less than 8.4 mg/dL), the patient may receive a higher dose of calcitriol or other vitamin D analog, a lower intake of the calcium receptor agonist, cinacalcet, or a greater intake of calcium to increase serum calcium levels. Serum calcium corrected for serum albumin may be calculated as follows (EQUATION 27.1) (11):
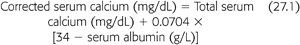
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


