Fig. 11.1
Operative positioning of the patient. The surgeon stands between the legs for most part of the operation
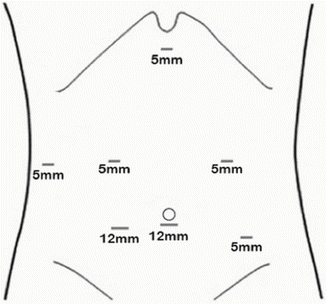
Fig. 11.2
Operative placement of ports. Seven ports are utilized and the camera alternates between the midline and right abdominal port. The left subcostal port is utilized for retraction of the gallbladder and the subxiphoid port is utilized for liver retraction
Instrumentation
High quality imaging systems are preferable. Routine hand instruments including bowel graspers and needle holders are also used. The special instruments, which are very useful for resection include ultrasonic shears (Ethicon), specialized bipolar coagulation probes, endoscopic gastrointestinal (GIA) linear staplers, and laparoscopic ultrasound probes (LUS) with 7–10 MHz frequency.
Technical Description
The technique of LPD is similar to that of open surgery. It involves the three phases: (1) laparoscopic staging and assessment of resectability, (2) resection, and (3) reconstruction.
Phase I
Laparoscopic staging of periampullary and pancreatic lesions is entirely different from the conventional method. The tactile sensation of the open method is replaced by LUS and Doppler examination. It is important to get adequate exposure and various measures for exposure include cranial traction of the gallbladder, right-sided table tilt, tacking the falciform ligament to the anterior abdominal wall, and mobilization of the right colon and hepatic flexure. Initially, any free fluid in the peritoneal cavity is aspirated and sent for cytology. Then a systematic examination of the peritoneum is performed and suspicious deposits are biopsied. Specific areas that should be examined include the sub-diaphragmatic regions, the falciform ligament, and the pelvis. Then, a systematic inspection of the liver is performed on all surfaces, which is facilitated by reverse Trendelenburg and left lateral tilt of the table. The gastrohepatic omentum overlying the caudate lobe of the liver is opened thereby exposing caudate lobe, celiac axis, and inferior vena cava. The hepatic artery is also visualized. Portal, perigastric, and celiac axis lymph nodes are biopsied if enlarged.
LUS is performed to evaluate small hepatic lesions (<1 cm) that are not usually identified by CT scan. All segments of the liver are examined sequentially by moving and rotating the probe slowly. The hepatoduodenal ligament is evaluated by placing the probe transversely. The portal vein and superior mesenteric vein (SMV) and artery (SMA) are examined and their relation to the tumor is assessed. The pancreas is examined by placing the transducer through the window in the gastrohepatic and gastrocolic omentum directly onto the surface of the gland. Gentle rotation of the probe at this stage allows for evaluation of the celiac axis and proximal hepatic artery.
Division of the gastrocolic trunk opens up the anterior aspect of the SMV to approach the plane between the neck of the pancreas and SMV. Tunneling behind the neck is safely performed using blunt irrigation and a suction instrument (Fig. 11.3). Resectability can be easily assessed in early periampullary lesions but may be difficult with pancreatic head carcinoma, particularly in association with chronic pancreatitis when LUS cannot accurately assess and stage the disease.
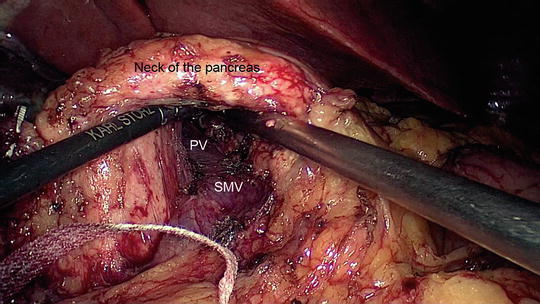

Fig. 11.3
Dissection of the neck of the pancreas away from the superior mesenteric vein (SMV) and portal vein (PV). The pancreas is lifted in the air and an umbilical tape will be placed around the neck
Phase II
Mobilization of the Pancreatic Head
To start, Cattell-Braasch maneuver is performed followed by an extended Kocher maneuver. The Cattell-Braasch maneuver is best performed by standing on the left side of the patient; and surgeon moves to right side of the patient for kocherization. The colo-hepatic peritoneum is incised and the hepatic flexure is mobilized down. This exposes the entire second and third part of the duodenum up to the neck of the pancreas. Kocherizing the duodenum is facilitated by retracting it anteriorly and medially by an atraumatic bowel grasper through the subxiphoid port. The duodenum and pancreas are dissected free to the left border of the aorta (Fig. 11.4). The right gastroepiploic vein and artery are clipped and divided and the first portion of the duodenum is skeletonized. On the supra-pyloric side, the right gastric artery is divided thus freeing the pylorus and the first portion of the duodenum. Depending on the nature of the tumor and oncological requirement, either the pylorus or first portion of the duodenum is divided using an endo-GIA stapler.
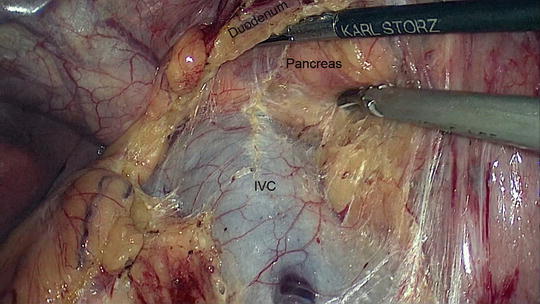

Fig. 11.4
Kocherization of the duodenum. The inferior vena cava (IVC) is clearly seen along with the posterior aspect of the head of the pancreas
The dissection of portal structures begins with decompression of the gallbladder as it provides better visualization of the structures in the porta hepatis. Calot’s triangle is dissected and the cystic artery and duct are clipped and divided. The gallbladder is left attached to the liver until the reconstruction is over as it provides good retraction of the liver. The common hepatic duct is transected above the level of the cystic duct junction and all fibro-fatty and lymphatic tissues along the hepatoduodenal ligament are cleared exposing the proper hepatic artery and portal vein. Bile spillage from the cut end of the hepatic duct is avoided by applying an endo-bulldog clamp. The hepatic artery is then traced along the superior border of the pancreas in the gastrohepatic omentum where the gastroduodenal artery may be identified and ligated near its origin from the common hepatic artery (Fig. 11.5). Dissection is continued towards the celiac axis along the superior border of pancreas taking lymphatic tissues with the specimen.
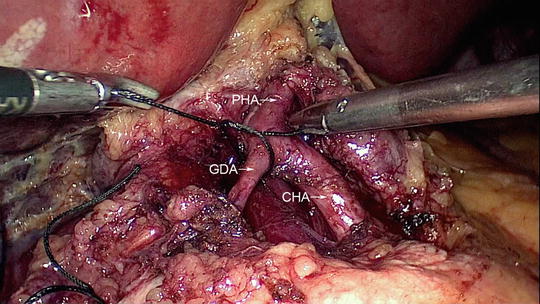

Fig. 11.5
Ligation of the gastroduodenal artery close to its origin from the common hepatic artery (CHA). The proper hepatic artery (PHA) is also identified
Infra-Colic Dissection
The peritoneum lateral to the duodenojejunal flexure is incised. The inferior mesenteric vein is carefully retracted to the left and the ligament of Treitz is divided. The jejunum about 10 cm distal to the ligament of Treitz is divided using an endoscopic GIA stapler. Jejunal vessels of the proximal jejunum and duodenum are divided using ultrasonic shears (Ethicon). Care should be taken while dividing the proximal jejunal tributaries that drain to the SMV posterior to the superior mesenteric artery (SMA). These jejunal branches also receive small tributaries from the uncinate process of the pancreas. Hence, extreme care should be taken in delineating these branches to avoid bleeding. The free end of the jejunum is delivered into the supracolic compartment.
Resection of Pancreas
The patient is tilted to the left in reverse Trendelenburg position, which facilitates exposure of the pancreas. The camera is placed in the right lateral port instead of the supra-umbilical port. Pancreatic transection at the neck begins after placing stay sutures at the superior and inferior borders on either side of the transection line to help in retraction and hemostasis. Pancreatic transection is performed using ultrasonic shears (Ethicon) while the area of the suspected pancreatic duct is divided with scissors to avoid injury to the ductal mucosa.
Resection of Uncinate Process
A hook retractor or an umbilical tape is applied across the root of the small bowel mesentery to give traction anteriorly and to the left. This maneuver clearly exposes the uncinate process to facilitate its resection and lymph node clearance along the right border of the SMA. The inferior pancreaticoduodenal artery is controlled and clipped by retracting the SMV medially. All other venous tributaries and small vessels of the uncinate process are identified separately, clipped, and divided. Great care is taken not to injure any of the major vessels (Fig. 11.6). Similarly, the pancreatic neck vein that drains posteriorly into the portal vein can be controlled between the common bile duct and portal vein. Following complete mobilization, the specimen is placed in an endoscopic specimen bag introduced through a 10-mm port and is left in the upper quadrant.
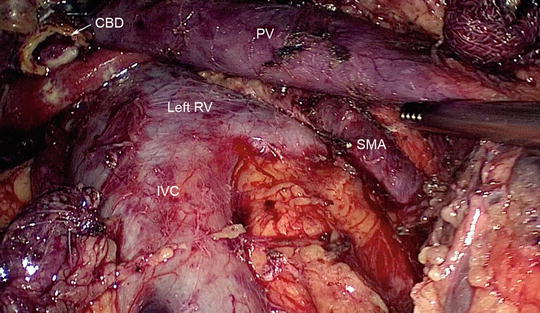

Fig. 11.6
At the completion of resection, the major vessels should be visible. The inferior vena cava (IVC), left renal vein (RV), portal vein (PV), and superior mesenteric artery (SMA) are clearly identified. The open common bile duct (CBD) is also visible
Phase III
Pancreaticojejunal Anastomosis
The divided end of the jejunum is brought up to the supracolic compartment in a retrocolic fashion. The pancreatic stump is mobilized for a length of 2–3 cm to facilitate the pancreatic anastomosis. Pancreaticojejunal reconstruction is done by end-to-side duct-to-mucosa technique using 4-0 polydioxanone sutures (Ethicon). The technique begins by placement of seromuscular sutures from the jejunum to the posterior pancreatic capsule using interrupted 3-0 polypropylene sutures. This places the jejunal loop in alignment with the pancreatic stump, which facilitates the subsequent pancreatic duct-to-mucosa anastomosis to be tension free. Six to eight interrupted duct-to-mucosa sutures are placed starting at the 6’o clock position and continued on either side (Fig. 11.7). The knots are placed preferably outside the lumen to prevent obstruction to the flow of pancreatic juice. The anterior seromuscular sutures are then placed to complete the anastomosis. Stenting the anastomosis is optional and may be preferred in cases of a non-dilated pancreatic duct. If the pancreas is soft and the duct is small, a dunking or invagination technique may be performed.
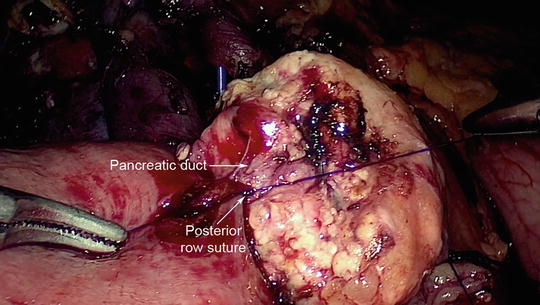

Fig. 11.7
Duct-to-mucosa anastomosis of the pancreaticojejunostomy. The posterior row suture has been placed
Hepaticojejunostomy
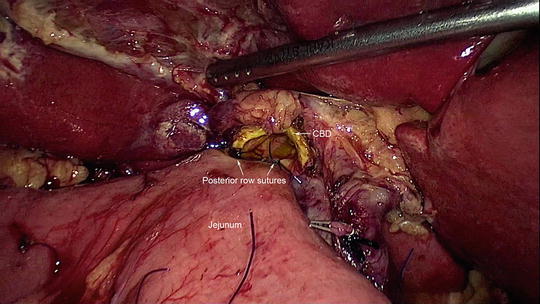
Approximately 7–8 cm distal to the pancreaticojejunal anastomosis, the hepaticojejunostomy is performed by creating a small enterotomy in the jejunum and anastomosing it to the cut end of hepatic duct. It is a single-layer anastomosis with 4-0 polydioxanone sutures (Ethicon) placed in interrupted manner starting on posterior border (Fig. 11.8). It is easier to perform the anastomosis from right to left for the posterior layer with the knots placed inside the lumen. Anteriorly, the sutures are placed sequentially from the corners to the center. If the duct is dilated, the anterior layer can be completed using a continuous suture. The anastomosis is better performed with the surgeon standing on the left side of the patient.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








