4
Laboratory Assessments: Implications in Diagnostics for the Andropause
Case History
A 32-year-old man presented with depression, loss of libido, and extreme tiredness. He would fall asleep at work, but had difficulty sleeping at night. He was concerned that his testosterone levels were falling after reading about this possibility in a men’s magazine. He said that he actively worked out and had a steady relationship. His exercise regimen included running in marathons and weight lifting as well. Physical examination revealed a well-built 6-foot-tall male weighing 180 pounds. His blood pressure was 120/80mmHg. He had no overt signs of hypogonadism, and was hairy in his chest. His gonads were of normal size. His laboratory results were as follows:
Total testosterone = 590ng/dL (260–1000ng/dL)
Free testosterone by dialysis equilibrium method = 32pg/dL (34–194pg/dL)
Bioavailable testosterone by ammonium precipitation method = 65ng/dL (84–402ng/dL)
Luteinizing hormone (LH), immunoassayed = 3.8mIU/mL (1.5–9.3mIU/mL)
Follicle-stimulating hormone (FSH), immunoassayed = 2.5mIU/mL (1.4–8.1mIU/mL)
Prolactin = 4ng/mL (2–18ng/mL), thyroid-stimulating hormone (TSH) = 1.38mIU/mL (0.4–5.5 mIU/mL)
Cortisol levels and magnetic resonance imaging (MRI) of the pituitary were normal, and sperm sample was normal; the patient was given a clomiphene challenge of 100 mg for 5 days, and his total testosterone went to 980ng/dL.
Different Tests to Measure Testosterone
Many clinicians and laboratories are confused as to the correct test to use when determining androgen status in aging men. Although opinion leaders have agreement in some areas as to which test to use, there are also areas of disagreement. This is in part because symptoms of androgen deficiency are not proportional to androgen levels and also because of the pulsatile nature of secretion of hormones. In any event, it is important for the practitioner to determine which test to order based on the clinical assessment of the patient.
Most laboratories measure the three domains of testosterone: total testosterone, free testosterone, and bioavailable testosterone. Fig. 4–1 represents the different fractions of testosterone. Total testosterone refers to all the testosterone that is measurable including those bound and unbound portions. Testosterone is bound to proteins like albumin and sex hormone–binding globulin (SHBG). As mentioned, changes in protein concentrations can alter the true levels and give false impressions. Testosterone is loosely bound to SHBG, and as such comes off easily, making it “free.” The actual free amount and that bound to SHBG are referred to as bioavailable testosterone. Laboratories can measure free testosterone using analog ligand radioimmunoassay methods, or they can sometimes calculate it based on a formula.
In older men, the binding of testosterone to SHBG is increased, making it less likely for it to be released and become free testosterone. As such, total testosterone in older men is much less reliable, and bioavailable testosterone is recommended instead. Bioavailable testosterone represents the “active form” of testosterone, and has a satisfactory correlation with androgenicity. It is also reduced more rapidly than total testosterone and as such approaches a real-time view of the androgen status of the patient. Unfortunately, most laboratories charge more for this test as it is more difficult to perform.
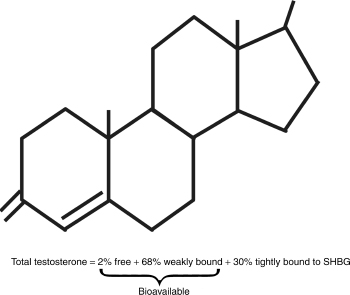
FIGURE 4-1. Fractions of testosterone.
Different Methods of Assays
Equilibrium dialysis is based on the separation of molecules of interest from solutions containing a mixture of cells and cellular products. Because of its physical simplicity, equilibrium dialysis is one of the key tests used in the study of protein binding. For equilibrium dialysis, neither fluorescent nor radioactive tags are needed. It is difficult to distinguish between bound and free ligand (small ions/molecules) in any mixture of ligand and macromolecule; therefore, equilibrium dialysis uses an indirect method to estimate the amount of ligand bound to a macromolecule. If, however, the free ligand can be dialyzed through a membrane until its concentration across the membrane is at equilibrium, the free ligand concentration can be measured easily.1 To perform an equilibrium dialysis, two half-cells of equal volume separated by a semipermeable dialysis membrane are used. This membrane is typically made of polysulfones or cellulose materials. Important system parameters are the pore size (of the membrane) and spatial distribution of the pores across the silicon substrate, as well as adhesion to the silicon surface.2 The dialysis is continued until equilibrium is reached and the concentration of free ligand is the same in both chambers. Because a portion of the ligand and macromolecule bind and are prevented from diffusing across the membrane into the assay chamber, the amount of free ligand in the assay is markedly reduced, and additional tests are required to provide information about specific binding sites. Although equilibrium dialysis is one of the more accurate methods, it is very time consuming.
Ultracentrifugation, on the other hand, is a technique that utilizes speed and sedimentation to separate macromolecules from solution. This process uses the approaches of sedimentation velocity and sedimentation equilibrium to characterize several macromolecular components. Properties such as homogeneity, macromolecular self-association, and macromolecule hydrodynamic properties (size, shape, and hydration) are ascertained through sedimentation velocity. Sedimentation equilibrium experiments, however, are conducted at significantly slower speeds “so that sedimentation is balanced by diffusion, yielding an equilibrium radial distribution of the macromolecule.”3 From this method, molecular weight, stoichiometry, and equilibrium association constants are obtained. The particle immersed in liquid within the centrifuge tube is acted on by three forces: buoyant force, the frictional force between the particle and the liquid, and particularly the centrifugal force, which is proportional to the rotational rate of the rotor and the distance of the tube from the center of the rotor.4 Reaching rotational speeds as high as 80,000 revolutions per minute (rpm), inert substances such as sucrose or cesium chloride, in which the concentration and density of the solution increase from top to bottom of the centrifuge tube, are often recommended for use in this procedure.5 When using the ultracentrifugation method, time and temperature of centrifugation must be carefully monitored. Control of time of centrifugation is important because a pellet at the bottom of the tube can form or insufficient separation can occur. Incorrect temperature settings can yield sample degradation or cause an increase in viscosity of solution and affect aggregation.6 One must also take note that with this method it is hard to assess purity of organelle preparations.
Radioimmunoassay (RIA) has been proven to be one of the most accurate tests and can be used to separate and measure even the smallest of molecules. In a typical RIA, the substance to be measured—the unlabeled sample antigen (also known as the ligand or analyte)—competes with radiolabeled antigen for a limited number of antibody binding sites.7 Radioisotopes such as tritium (3H), 57Co, and the radioactive isotope of iodine (125I) are used to tag proteins and peptides; however, because 125I (which emits gamma radiation as it decays) attaches easily to most antibodies or antigens, it is most commonly used as an isotopic tag. 125I also has a shorter half-life (60 days) as compared with tritium’s half-life of 12.5 years, and thus, relieves the expenses of disposal. For this test to work, both a radiolabeled analog of the ligand and pure form of the liquid must be used. Both the labeled and unlabeled antigens are equally probable of binding to the antibody, and reflect the amount of each present; therefore, the amount of radioactivity present is inversely proportional to the amount of sample antigen present. When the Ag:Ab complex precipitates out of solution, a radiolabeled isotope is added and allowed time to bond. It is then separated from unbound reagents and measured in a gamma counter.8 To determine the concentration of the unknown sample, it is compared with a standard curve in which the proportion of labeled to unlabeled antibody is known. The immunoradiometric assay (IRMA), also known as the sandwich method is a variation of the RIA method.7 This technique uses two antibodies that bind to the antigen at two different sites: a capture antibody bound to a solid phase (typically a glass or plastic bead), and an antibody labeled with 125I. These antibodies bind to the antigen and form the Ab-Ag-Ab(125I) sandwich. The unbound reagents are washed away, and radioactivity measured. Because IRMA radioactivity signal is directly proportional to the amount of analyte, it is often more accurate with lower concentrations of the analyte; the IRMA method, however, fails to perform well with smaller peptides, as their size permits only one binding site. Health hazards and the costs of complying with the handling and disposal regulations of the radioisotope have caused researchers to seek alternative labeling methods such as chromogenic, fluorescent, or luminescent tagging. These nonisotropic methods, however, have not been able to acquire the high sensitivity and specificity of the RIA.
Total Testosterone
Most laboratories use automated machines to determine total amounts of testosterone. To measure total testosterone, these instruments must first displace bound testosterone from SHBG and albumin. Usually low pH buffers, surfactants, salicylates, or a competing steroid that does not bind to antitestosterone antibody is used in the immunoassay. However, the testosterone antisera used in commercial preparations often cross-react with other steroids including dihydrotestosterone (DHT). Solvent extraction and chromatography have been used to remove these interfering compounds prior to testosterone measurement. Unfortunately, these cannot be incorporated into the methods used by automated analyzers. Fortunately, the plasma levels of DHT are only ∼10% of testosterone levels and cross-reactivity is usually less than 5%. Thus, in clinical application, the impact of DHT is minimal in most instances.
“Free” Testosterone
It has been generally agreed that free testosterone rather than total testosterone gives a better measure of androgenicity.9 However, different laboratories may report free testosterone using various methodologies. It is prudent that the clinician be aware of the different methods of obtaining a free testosterone level, and interprets it in the context of the patient. Testosterone circulates in plasma and binds to SHBG and albumin. Testosterone binding to transcortin and orosomucoid is negligible. Albumin-bound testosterone is released into the plasma easily as compared with those bound to SHBG. There are several measures of free and bioavailable testosterone:
Apparent Free Testosterone Concentration (AFTC) as Measured by Equilibrium Dialysis
Apparent free testosterone concentration (AFTC) or testosterone as determined by equilibrium dialysis at 37°C is arguably the method of choice for measuring free testosterone in vivo. The measurement of free testosterone in the serum is technically demanding, as the free testosterone concentration is very low (2%). Routinely available assays are not sensitive enough to quantify free testosterone directly. Usually, free testosterone is estimated by indirect methods. In these indirect methods, titrated testosterone is added to the sample and allowed to come to equilibrium with testosterone in the serum at a physiological temperature (37°C). The amount of added radiolabeled testosterone must be low enough to guarantee that addition will not significantly increase the total testosterone concentration. When equilibrium is achieved, the free testosterone is separated from the bound by filtration through a membrane. The filtration is accomplished by equilibrium dialysis or centrifugal ultrafiltration. The radioactivity of the protein-free ultrafiltrate is measured and used to calculate the percentage of free testosterone. The concentration of free testosterone can then be calculated by multiplying the percentage of free testosterone by the total testosterone concentration. Measurement of free testosterone by these methods is not available in most clinical laboratories due to the complicated nature of the testing and the requirement of a scintillation counter to measure the titrated testosterone concentration. Overall, the results of equilibrium dialysis and centrifugal ultrafiltration methods have been shown to be quite comparable. Although equilibrium dialysis is often considered to be the “gold standard,” centrifugal ultrafiltration is somewhat simpler to perform and may theoretically be more accurate due to the fact that the equilibrated sample is not diluted with dialysis buffer.
Free Androgen Index (FAI)
The concentration of testosterone in the different free and bound forms is really a function of total testosterone concentration and the relative concentrations of SHBG and albumin. It can be predicted that increased SHBG will decrease the concentration of both free and bioavailable testosterone for a given total testosterone concentration. Many clinicians use a calculated free androgen index to estimate physiologically active testosterone. This index is typically calculated as the ratio of total testosterone divided by SHBG and multiplied by 100 to yield numerical results comparable in free testosterone concentration. Otherwise, more complicated mathematical algorithms can be used to approximate the percentage of free testosterone from the SHBG concentration alone or in combination with albumin concentration. The precision of these algorithms is subject to the combined errors of the individual tests performed.
Direct Immunoassay of Free Testosterone with a Labeled Testosterone Analog (aFT)
Several commercial kits are available for the direct estimation of free testosterone in serum. These kits use a labeled testosterone analog that has a low binding affinity for both SHBG and albumin, but is bound by antitestosterone antibody. Because the analog is unbound in the plasma, it competes with free testosterone for binding sites on an antitestosterone antibody that is immobilized on the surface of the well or assay tube. The first kits developed used a radiolabeled testosterone analog to compete with free testosterone for binding sites on an antibody-coated polypropylene tube. More recently developed kits employ an enzyme-labeled analog that can be measured after competitive binding to antitestosterone antibodies coated to microtiter wells. These analog methods are technically less demanding than equilibrium dialysis or centrifugal ultrafiltration and require substantially smaller blood samples. The analog methods also offer the benefit of direct estimation of free testosterone concentration without the need to measure total testosterone. Many laboratories can readily perform the enzymatic methods because they are nonisotopic.
Winters and colleagues10 have found the analog method to correlate better with total testosterone levels than with bioavailable testosterone determined by the ammonium sulfate precipitation method. They suggested that the analog free testosterone results might be misleading in men with low SHBG concentration. Fig. 4–2 shows the correlation of total testosterone with bioavailable and free testosterone.
Ooi and Donnelly11 suggested that the problems observed by Winters et al might be resolved, in large part, simply by using a more appropriate population-based reference interval. Vermeulen and colleagues12 found that the analog-free testosterone method correlated well with free testosterone by equilibrium dialysis but did not correspond with a free testosterone calculated from total testosterone and SHBG.
Free testosterone (FT) can also be calculated from total testosterone and immunoassayed SHBG.
Bioavailable Testosterone (BT)
Bioavailable testosterone (BT) is the fraction of serum testosterone not precipitated by 50% ammonium sulfate concentration. As in the free testosterone methods described previously, titrated testosterone is added to serum that is then allowed to come to equilibrium at physiological temperature. Testosterone bound to SHBG is then selectively precipitated with 59% ammonium sulfate, leaving free and albumin bound testosterone in the solution. The percentage of titrated label not bound to SHBG is multiplied by the total testosterone to produce the bioavailable testosterone. Another method of measuring bioavailable testosterone is by direct radioimmunoassay in the supernatant after solvent extraction.
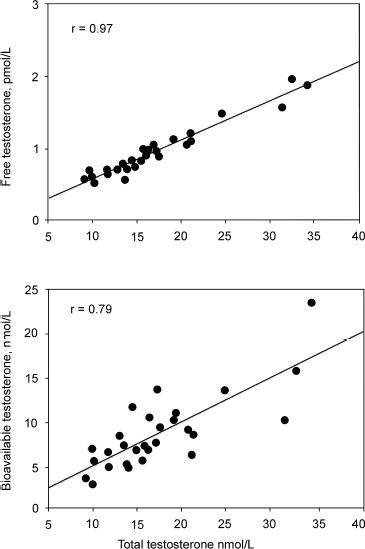
FIGURE 4-2. Winters et al study suggesting that the analog method of free testosterone rather than the bioavailable testosterone is better correlated to total testosterone. (Adapted from Winters SJ, Kelly DE, Goodpaster B. The analog free testosterone assay: are the results in men clinically useful? Clin Chem 1998;44:2178–2182, with permission.)
Care must be taken to ensure that the labeled tracer testosterone used for measuring the FT fraction is highly purified. In a study by Vermeulen et al,12 AFTC was correlated against the other measures of testosterone. A coefficient of correlation of 1.0 would mean a perfect correlation. In that study with men, they found that the correlation of AFTC with FT (calculated free testosterone) = 0.987, aFT (immunoassayed free testosterone) = 0.937, FAI (free androgen index) = 0.848. In other words, calculated free testosterone approaches the accuracy of measuring testosterone by dialysis equilibrium. It has to be noted that conditions that alter SHBG may alter the results of not only total testosterone but also FT. In men, conditions like obesity, hypothyroidism, and acromegaly can lead to lowered levels of SHBG, and as such confound the results of FT. Incidentally, pregnancy also leads to altered levels of SHBG, and as a result leads to false levels of FT as well. Otherwise, calculated free testosterone (FT) may be a practical means for the clinician to measure free testosterone, as it is less time consuming and expensive than testosterone by equilibrium dialysis (AFTC). Bioavailable testosterone is a more expensive test, but will be more useful and accurate in older patients as SHBG binding increases with age, and BT measures only the free amounts and those loosely bound to albumin. In older patients, one often finds normal levels of total testosterone, but BT is often significantly depressed.
Salivary Testosterone
This form of testing is novel, especially for the patient, as it avoids a needle stick. It is also convenient for the patient, as it does not require coming to the office. The patient spits into a bottle and mails the sample to the laboratory. Fig. 4–3 illustrates a typical kit. This test may be useful for screening for hypogonadism but not for a diagnosis of the andropause syndrome. The history and physical examination should be weighted more than salivary tests or any other blood tests. Saliva testosterone does not give a real-time assessment of the androgen status, and patients on androgen therapy often have levels of testosterone in the thousands. It gives a picture of accumulated testosterone. However, when done properly, saliva testosterone has good correlation to free testosterone (r = 0.90), less for total testosterone (r = 0.85). The correlation for dehydroepiandrosterone (DHEA) is less at 0.70, and androstenedione 0.74. The patient has to rinse his mouth 5 minutes before testing and avoid food and tooth brushing for 30 minutes. He chews on a gum and spits into a bottle and mails the specimen to the laboratory.

FIGURE 4-3. Saliva test kits. Patient typically spits into a test tube, which is mailed to a laboratory.
Dynamic Testing for Testosterone
HCG Stimulation Test
Human chorionic gonadotrophin (HCG) is a glycoprotein with physiological actions similar to those of LH. After an intramuscular injection of HCG, the hormone binds to the LH receptors in the Leydig cells, and stimulates the production and secretion of testosterone. Typically, the test dose is 4000 IU for 4 days. This test is used to assess the viability of the axis and whether there is a gonadal disease. A positive response usually results in doubling of testosterone levels and improvement in symptoms. An alternate dosing is 5000 I.U. and measuring testosterone levels 3 days later. If there is no response, it is indicative of testicular failure, and if there is a response, it is indicative of pituitary-hypothalamic failure.13
Clomiphene Citrate Test
Clomiphene is a nonsteroid oral compound with estrogenic effects. It binds to estrogenic receptors in the body, and the hypothalamus responds by secreting LH. The test dose is 50 to 100 mg b.i.d. for 10 days, and both testosterone and LH levels are measured. By and large, healthy men have a 50% to 200% increase in LH and testosterone levels. If there is no response, it is indicative of testicular failure, and if there is a response, it is indicative of pituitary-hypothalamic failure.13
Variability in Testosterone Production and Measurements
Hormones are in constant fluctuation because of the pulsatile nature of their release. A single value is often misleading for the clinician, although single determinations may help differentiate between normal individuals and patients with severe hypogonadism. However, frequent samplings of testosterone may be necessary to guide the practitioner for an accurate diagnosis of the andropause syndrome. Stress can also lead to transient lowering of testosterone and to misdiagnosis. Testosterone is bound to various proteins including albumin and SHBG. As such, variations in concentrations of SHBG, for example, can alter levels of testosterone. Conditions that can lower SHBG concentrations include hypothyroidism, obesity, and acromegaly.
Spratt et al14 studied the neuroendocrine gonadal axis in 20 men by frequent sampling of LH, FSH, and testosterone (Figs. 4–4 and 4–5). They found that in normal men, serum total testosterone concentrations determined at 6-hour intervals ranged from 105 to 1316 ng/dL between subjects. When testosterone was measured at 20-minute intervals, marked intermittent declines in testosterone concentrations to levels well below the normal range were observed in 3 of the 10 subjects. The authors noted that testosterone secretion lagged behind LH secretion by ∼40 minutes (p < .02). This suggests that LH’s influence on Leydig cells is not immediate. LH in itself had great variability as well. Both mean LH concentrations and mean LH pulse amplitudes varied fourfold between individuals. LH interpulse intervals also varied from 30 to 480 minutes. The results suggested that there is a relative refractory period at the level of the hypothalamus or pituitary. In some subjects, there was a striking nighttime accentuation of LH, which is not seen with FSH.
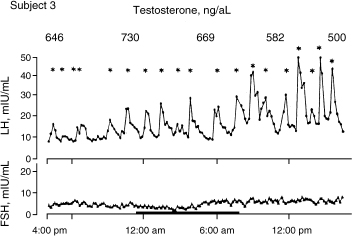
FIGURE 4-4. Variability of luteinizing hormone (LH) compared with follicle-stimulating hormone (FSH) in a 24-hour period in subject. (Adapted from Spratt DI, O’Dea LS, Schoenfeld D, et al. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH and testosterone. Am J Physiol 1988;254: E658–666, with permission.)
In another study, Morley et al15 compared the results of various testosterone assays in a cross-sectional study of 50 men age 28 to 90 years. The purpose of the study was to determine the relationship of the various testosterone assays to one another. In addition, they also determined the week-to-week variability in testosterone and bioavailable testosterone in 16 subjects. Hypogonadism may be diagnosed too frequently, and perhaps inappropriately especially in older men, due to an increase in their SHGB levels. There are often errors in the assays available to measure testosterone, thus leading to discrepancies in diagnosing hypogonadism. These tests include a total testosterone test, free testosterone test (by dialysis and ultracentrifugation), bioavailable testosterone, direct measurement of free testosterone by an analog immunoassay, and calculated free testosterone via a free androgen index (FAI) and a free testosterone index (FTI). Although measurement of free testosterone by equilibrium dialysis at 37°C (AFTC) is time-consuming, it is regarded as the best method for estimation of free testosterone, and is thus used as the “gold standard” in Morley’s investigation. In the study, results of different testosterone tests were compared to determine the more accurate tests. First, blood samples from each subject were taken between 8 a.m. and 10 a.m. At the time of blood draw, none of the subjects was known to by hypogonadal, and none had disease or took medications that produce a decline in testosterone. None had an elevated LH level. No exclusion was made based on the serum SHBG level. The following tests were performed on each sample: the total serum testosterone, serum SHBG, and free testosterone by dialysis, bioavailable testosterone tests by radioimmunoassay (via a commercial available kit), and free testosterone by ultracentrifugation (via the method of Ekins). An analog radioimmunoassay was used to directly estimate the serum free testosterone. The FAI was calculated (total testosterone/SHBG), and the FTI was calculated using the method of Vermeulen et al.12 A false-positive test meant that the subject was identified as hypogonadal by total testosterone, but eugonadal by BT or AFTC. A false negative represented the subject’s classification as eugonadal by total testosterone, but hypogonadal by BT or AFTC. Using 300ng/dL as a cutoff point in defining hypogonadism, the authors discovered that 42% of the men were misclassified using the total testosterone test. Similar results were observed when comparing total testosterone and AFTC. When comparing total testosterone with BT, total testosterone produced 16% false positives and 26% false negatives; however, a week-to-week comparison showed that BT varied more than total testosterone. Over an 8-week period, BT identified 10 of 16 men as hypogonadal at one point and eugonadal at another point, whereas in the total testosterone test, 8 of 16 men were considered hypogonadal at one point and eugonadal at another point. When comparing free testosterone by dialysis (FTD) with BT, there were 6% false positives and 30% false negatives. The final assessment of the tests show that aFT (the analog immunoassay procedure) corresponded with free testosterone and BT measurements, but did not correlate with AFTC. Also, although FAI connects with AFTC, the FAI/AFTC ratio is significant only in low levels of SHBG; thus, FAI may not be very accurate in older men with high SHBG. Morley et al’s results were also consistent with Vermeulen et al’s in that there was a close correlation between FTA and FTD and BT, making FTI a reliable measurement of unbound testosterone. Based on the discrepant results of total testosterone, the study concluded that either BT or some measure of free testosterone (AFTC, aFT, or FT) be used to determine hypogonadism; however, taking note of BT’s week-to-week variability, a second assay must be used 1 or 2 weeks apart in light of a normal reading on the first measurement.
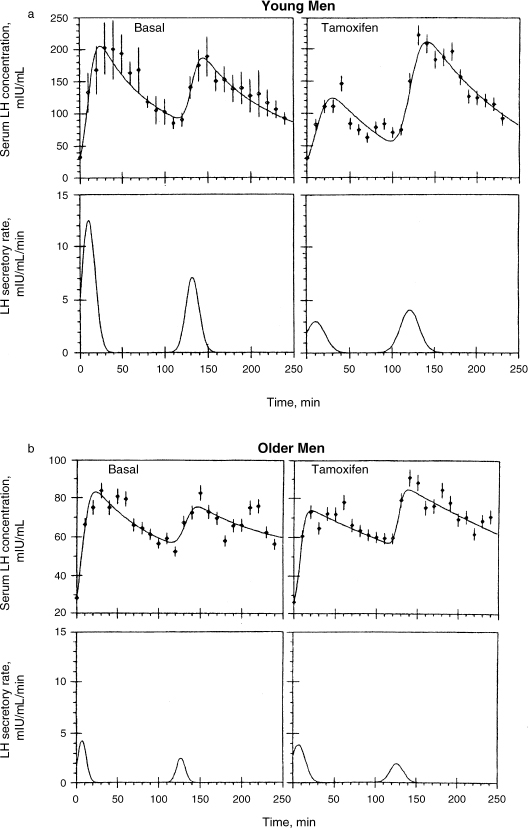
FIGURE 4-5. Augmentation of bioactive LH secretion and enhancement of bioactive LH and enhancement of plasma bioactive/immunoreactive ratios by antiestrogens (tamoxifen) were significantly reduced or absent in older men. (Adapted from Spratt DI, O’Dea LS, Schoenfeld D, et al. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH and testosterone. Am J Physiol 1988;254:E658–666, with permission.)
Measuring Luteinizing Hormone and Follicle-Stimulating Hormone in Aging Men
LH and FSH are glycoprotein gonadotrophins composed of α and β subunits secreted by the same cell. The biological activity of HCG, which is a glycoprotein from the placenta, resembles that of LH. LH and FSH bind to receptors in the testes and regulate gonadal function by promoting sex steroid production and gametogenesis. LH stimulates testosterone production from the interstitial cells of the testes (Leydig cells). Maturation of spermatozoa requires both LH and FSH. The secretion of LH and FSH is episodic with secretory bursts every hour and are mediated by a concomitant episodic gonadotrophin-releasing hormone (GnRH) release. Arguably, FSH is less pulsatile than LH and is sometimes used by clinicians as a measure of gonadal failure with aging. Testosterone is not the sole inhibitor of gonadotrophin secretion in men, as selective destruction of the testes by chemotherapy results in azoospermia and a rise in FSH only. Inhibin, which is secreted by the Sertoli cells of the seminiferous tubules, is the major factor that inhibits FSH secretion by negative feedback.
By and large, LH rises with aging and there is a concomitant drop in bioavailable testosterone levels. However, it is to be noted that the rise in LH seems to be inconsistent in aging men. In younger men, gonadal failure leads to fairly consistent rises in LH, partly because the hypothalamic-pituitary-testicular axis is intact. With aging, this system is impaired and results in inconsistent rises. The disparity of low bioavailable testosterone and yet normal or low LH is consistent with a hypothesis of a relative hypogonadotropism, often seen in the andropause syndrome.
Urban et al16 reported that some healthy older men exhibited evidence of neuroendocrine dysfunction, reflected by irregular bursts of bioactive LH release followed by transiently low plasma bioactive/immunoreactive (B/I) ratios. It has been reported that mean basal plasma bioactive LH concentrations, B/I ratios, and spontaneous LH pulse properties (peak, frequency, amplitude, duration, and enhanced B/I ratios within LH peaks) were not altered in older men. On the other hand, augmentation of bioactive LH secretion and enhancement of bioactive LH and enhancement of plasma B/I ratios by pulsed injections of exogenous GnRH were significantly reduced or absent in older men. In that study, authors conclude that bioactive LH reserve is markedly attenuated in older men challenged with either exogenous GnRH or antiestrogens.
In another study, Veldhius et al17 explored an andropause state using ketoconazole, which is an antiandrogen. This study found a discrepancy between young and older men. In young men, the chemically induced andropausal state resulted in an elevated LH peak frequency, whereas there was a reduced incremental LH pulse area in older men. This study also supported the earlier study in that older men had an impoverished augmentation of LH pulse mass, impaired orderliness of LH release, and diminished 24-hour rhythmic LH secretion. Mitchell et al18 also reported that there were age-related changes in the pituitary-testicular axis in normal men. However, they pointed out in their study that immunoreactive LH remained unchanged despite finding that levels of total testosterone and bioactive LH fell with age. The important lesson from this study is that as men age, there is a hypothalamic-pituitary defect that in turn leads to lower bioactive LH levels, which in turn is responsible for diminished gonadal steroidogenesis. In yet another study by Veldhius et al,19 they found that age was a negative determinant of LH secretory burst amplitude and a positive predictor of LH secretory burst frequency as well as basal LH secretory rates. They suggested the attenuation of LH secretory burst amplitude as an approximate basis for hypoandrogenism of healthy aging in older men.
Should the clinician measure LH levels to determine if the patient has indeed undergone andropause? The LH surge in women undergoing menopause is not seen in men. If the LH levels were very high in older men, a pituitary cause for hypogonadism must be excluded. Often, men with andropausal symptoms have LH levels within the normal range (0.5–15 ng/dL), but have low bioavailable testosterone. As such, the use of LH is more useful in excluding diagnosis of other pathological states. When ordering LH, the clinician should distinguish between bioavailable and immunoreactive LH. Some laboratories are not able to provide both tests, and do only the immunoreactive LH. Overall, LH tends to be secreted in spurts as well, and it is difficult to rely on single values. FSH is the gonadotrophin that stimulates spermatogenesis in the Sertoli cells. It has been suggested that FSH is less pulsatile, and that it may be a better measure of gonadal failure as a result of aging in men. This is yet to be verified in clinical trials.
Measuring Estrogens in Men
In recent years, the role of estrogens in men has become more important because of the discovery of human models of estrogen deficiency such as estrogen resistance or aromatase deficiency.20 In men, testosterone remains the major source of plasma estradiol. The main biologically active estrogen is estradiol. The testes secrete 20% of men’s estradiol. On the other hand, plasma estrone (5% of which is converted to plasma estradiol) originates from tissue aromatization of mainly adrenal androstenedione. The plasma concentration of estradiol in older men is ∼20 to 30pg/mL and its production rate in blood is 25 to 40µg/24 hour. It is interesting to note that both of these values are actually significantly higher than in postmenopausal women. Plasma levels of estradiol do not necessarily reflect tissue-level activity, as peripherally formed estradiol is partially metabolized in situ. Not all enters the general circulation, with a fraction remaining only locally active. Of the factors influencing plasma estradiol levels, plasma testosterone remains most important. However, the age-associated decrease in testosterone levels is scarcely reflected in plasma estradiol levels, as a result of increasing aromatase activity with age and the age-associated increase in fat mass. Free and bioavailable estradiol levels decrease modestly with age as does the ratio of free testosterone to free estradiol. Estradiol levels are highly significantly positively related to body fat mass and more specifically to subcutaneous abdominal fat, but not to visceral (omental) fat. Indeed, aromatase activity in omental fat is only one-tenth of the activity in gluteal fat. Estrogens in men play an important role in the regulation of the gonadotropin feedback, cognitive functions, bone maturation, regulation of bone resorption, and lipid metabolism. Estrogens also affect skin metabolism and are important determinants of sexual interest in men.
Low Testosterone in Athletes/Receptor Issues
For years researchers have known that exercise training in women often affect women’s sex hormone levels. Exercise training in women produces various degrees of menstrual alterations, ranging from obvious clinical forms such as amenorrhea, to less obvious subclinical forms, such as luteal phase defects, or anovulation.21 Monitoring sex hormone levels in males is more difficult; however, recent studies have shown male endurance-trained athletes (particularly runners) may experience similar hormone problems. These changes include a reduction in total and free testosterone, alterations in LH release, alterations in pituitary responses to GnRH, and other pharmacological perturbations.22
Leydig cells, which are regulated by the pituitary hormones FSH and LH, produce androgens such as testosterone, dihydrotestosterone, androsterone, androstenedione, etc. Some studies have shown significantly lower levels of testosterone22,23 and free testosterone24,25 in endurance-trained men compared with those of age-matched sedentary controls22; however, other researchers have not been able to support these results.26–29 Investigators have also tried monitoring LH and FSH levels. LH and FSH are released in an episodic manner from the pituitary gland in response to the pulsatile release of GnRH.30 Although researchers have again varied in their results, it is believed that discrepancies in results may lie in the variability in age, lifestyle, and type of exercise of the subjects. It must be clarified, however, that although some studies observed lower levels of free testosterone, these concentrations were still within the low normal range.
Prolactin is a stress hormone produced in the pituitary gland. Elevated prolactin levels (often found in male athletes) are detrimental to testosterone production, spermatogenesis, and fertility.31,32 Much of the available information is reflective of animal studies. On the basis of these animal studies, it has been suggested that physical exercise affects the dopaminergic, noradrenergic, and serotonergic systems.33–37 Hackney et al24 corroborated the effect of exercise on the dopaminergic system through experimentation with the dopamine antagonist, metoclopramide. Their results suggested that elevated dopamine levels in endurance-trained men are responsible for fluctuations in GnRH and prolactin release. Other scientists believe that the levels of stress-related hormones such as prolactin, and several endocrine components of the hypothalamic-pituitary-adrenal (HPA) axis, such as corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and cortisol, may participate in complex feedback mechanisms that disrupt the activity and release of hypothalamic GnRH and pituitary LH and FSH.38–40 Although some researchers have observed a decrease in testosterone, further long-term studies are needed to clarify and confirm results.
Discussion of Case History
The patient is seemingly hypogonadal based on his history and laboratory findings. However, his age and physical examination did not quite fit the picture of a true state of hypogonadism. He had no obvious trigger to push him into premature andropause, like chemotherapy, testicular tumor or surgery, mumps orchitis, or trauma. However, arguably, he has participated in marathon races, which can suppress testosterone levels. It was unusual that he should be in andropause. He was challenged with clomiphene to see if there was true Leydig cell failure, and there was not, as his testosterone levels went up quite quickly. He was not hypothyroid, and there was no evidence of a pituitary tumor. In the end, his symptoms were attributed to clinical depression. He had several samplings of his testosterone, and they normalized. He even had his 24-hour urinary free testosterone measured, which was in the normal range. He responded to a course of antidepressants, switching from Zoloft to Paxil and finally to Wellbutrin. This case highlights the variability of testosterone measurements and that before committing a patient to testosterone therapy, a clinician must consider other physiological and pathological causes for the symptoms.
Conclusion and Key Points
• Laboratory assessments should only supplement and not replace a clinical assessment of a patient suspected to have the andropause syndrome.
• There are different measures of free testosterone, and the dialysis equilibrium method (AFTC) is arguably most exact, and the calculated (FT) or free androgen index (FAI) approaches the accuracy of AFTC.
• Bioavailable testosterone is the free testosterone portion plus that bound to albumin, which is loosely bound, and is useful especially in older patients with SHBG issues.
• Measuring LH is useful in excluding diagnosis.
• Estrogens in men have a physiological role, and could affect the balance of testosterone.
• To offset the circadian rhythms of testosterone and the variability, it may be necessary to measure urinary free testosterone over a 24-hour period to determine hypogonadal status in some patients.
REFERENCES
1. Harvard/Amika International Studies. Introduction to equilibrium dialysis. 2001. http://www.harvardapparatus.com/pdffiles/B2K_N121.pdf
2. Turner J. Equilibrium dialysis systems for selective molecular filtration. Cornell Nanofabrication Facility. 2001. http://www.nnf.cornell.edu/2001cnfra/200156.pdf
3. Brenowitz M. What is analytical ultracentrifugation? Albert Einstein College of Medicine. June 19, 2002. http://www.bioc.aecom.yu.edu/labs/brenlab/XL-I/XL-I.html
4. Kanjee U. Ultracentrifugation to separate cellular components. JLM349S, Eukaryotic Molecular Biology at the University of Toronto. June 19, 2002. http://www.cquest.utoronto.ca/botany/bio349s/techniques/assessingcells/15ultracentr.html
5. Rickford D. Centrifugation. New York: John Wiley; 1994;1–100
6. Block R. Methods of Protein Chemistry. New York: Pergamon Press; 1991:119–173
7. Krumm R. Radioimmunoassay: a proven performer in the bio lab. The Scientist. May 16, 1994. http://www.the-scientist.com/yr1994/may/tool_940516.html
8. Smith R, Simpson B. Radioimmunoassay (RIA). Department of Veterinary Pathobiology College of Veterinary Medicine Texas A&M University. Aug. 29, 2002. http://vtpb-www.cvm.tamu.edu/vtpb/vet_micro/serology/ria/default.html
9. Valcour A. Testosterone: free T or not free T. Adv Lab 2001; 11:3–10
10. Winters SJ, Kelly DE, Goodpaster B. The analog free testosterone assay: are the results in men clinically useful? Clin Chem 1998;44:2178–2182
11. Ooi DS, Donnelly JG. More on the analog free testosterone assay. Clin Chem 1999;45:715
12. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672
13. Greenspan FS, Gardener DG. In: Basic and Clinical Endocrinology. 6th ed. New York: Lange Medical Books; 2001
14. Spratt DI, O’Dea LS, Schoenfeld D, et al. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH and testosterone. Am J Physiol 1988;254:E658–E666
15. Morley JE, Patrick P, Perry HM. Evaluation of assays to measure free testosterone. Metabolism 2002;51:554–559
16. Urban RJ, Veldhius JD, Blizzard RM, et al. Attenuated release of biologically active luteinizing hormone in healthy aging men. J Clin Invest 1988;81:1020–1029
17. Veldius JD, Urban RJ, Lizarralde G, et al. Attenuation of luteinizing hormone secretory burst amplitude as a proximate basis for the hypoandrogenism of healthy aging men. J Clin Endocrinol Metab 1992;75:704–706
18. Mitchell R, Hollis S, Rothwell C, et al. Age related changes in the pituitary testicular axis in normal men: lower serum testosterone results from decreased bioactive LH drive. Clin Endocrinol (Oxf) 1995;42:501–507
19. Veldhuis JD, Zwart A, Mulligan T, et al. Muting of androgen negative feedback unveils impoverished gonadotrophin releasing hormone/luteinizing hormone secretory reactivity in healthy older men. J Clin Endocrinol Metab 2001;86:529–535
20. Vermeulen A, Kaufman JM, Goemaere S, et al. Estradiol in elderly men. Aging Male 2002;5:98–102
21. Brocks A, Pirke KM, Schweiger U, et al. Cyclic ovarian function in recreational athletes. J Appl Physiol 1990;68: 2083–2086
22. Arce JC, De Souza MJ, Pescatello P, Luciano AA. Subclinical alterations in hormone and semen profile in athletes. Fertil Steril 1993;59:398–404
23. Ayers WT, Komesu Y, Romani T, Ansbacher R. Anthropomorphic, hormonal, and psychological correlates of semen quality in endurance-trained male athletes. Fertil Steril 1985;43: 917–921
24. Hackney AC, Sinning WE, Bruot BC. Reproductive hormonal profiles of endurance: trained and untrained males. Med Sci Sports Exerc 1988;20:60–65
25. Wheeler GD, Wall SR, Belcastro AN, Cumming DC. Reduced serum testosterone and prolactin levels in male distance runners. JAMA 1984;252:514–516
26. Bagatell CJ, Bremner WJ. Sperm counts and reproductive hormones in male marathoners and lean controls. Fertil Steril 1990;53:688–692
27. Gutin B, Alejandro D, Duni T, Segal K, Phillips GB. Levels of serum hormones and risk factors for coronary hear disease in exercise-trained men. Am J Med 1985;79:79–84
28. MacConnie SE, Barkan A, Lampman RM, Schork MA, Beitins IZ. Decreased hypothalamic gonadotropin-releasing hormone secretion in male marathon runners. N Engl J Med 1986;315: 411–417
29. Mathur N. Toriola AL, Dada QA. Serum cortisol and testosterone levels in conditioned male distance runners and non-athletes after maximal exercise. J Sports Med Phys Fitness 1986;26:245–250
30. Crowley WF Jr, Filicore M, Spratt DI, Santoro N. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res 1985;41:473–531
31. Ambrosi B, Gaggini M, Travaglini P, et al. Hypothalamic-pituitary-testicular function in men with PRL-secreting tumors. J Endocrinol Invest 1981;4:309–315
32. Perryman RL, Thorner MO. The effects of hyperprolactinemia on sexual and reproductive function in men. J Androl 1981; 2:233–242
33. Brown BS, Van Huss WD. Exercise and rat brain catecholamines. J Appl Physiol 1973;34:664–669
34. Brown BS, Payne T, Kim C, et al. Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J Appl Physiol 1979;46:19–23
35. Chaoloff F. Physical exercise and brain monoamines: a review. Acta Physiol Scand 1989;137:1–13
36. Chaoloff F, Laude D, Serrurier B, et al. Brain serotonin response to exercise in the rat: the influence of training duration. Biog Amines 1987;4:99–126
37. Elam M, Svenson TH, Thoren P. Brain monoamine metabolism is altered in rats following spontaneous, long-distance running. Acta Physiol Scand 1987;130:313–316
38. Martini L. The 5 alpha-reduction of testosterone in the neuroendocrine structures: biochemical and physiological implications. Endocr Rev 1982;3:1–25
39. Martini L, Zoppi S. Mode of action of androgens in neuroendocrine structures. In: Paulson et al, eds. Andrology: MaleFertility and Sterility. London: Academic Press; 1986:149–159
40. Negro-Vilar A, Valenca MM. Male neuroendocrinology and endocrine evaluation of reproductive disorders. In Lamb J et al, Eds. Physiology and Toxicology of Male Reproduction. San Diego: Academic Press; 1988:103–131
< div class='tao-gold-member'>









