Peripheral Nerve Evaluation
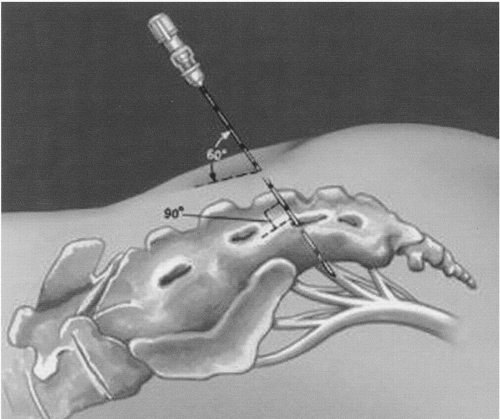
When initially marketed, the initial test phase was called the peripheral nerve evaluation (PNE). With the advent of the tined lead, this phase is less commonly done and therefore will be described only briefly. In the office, the patient is placed in the prone position with pillows under the abdomen to flatten the back and under the knees to elevate the lower limbs. The skin and subcutaneous tissue and periostium are infiltrated with a Xylocaine or Xylocaine/bicarbonate solution (1% Xylocaine with 8.4% bicarbonate in a ratio of 10 to 1) over the S3 foramen. The addition of bicarbonate decreases stinging and burning from the highly acidic Xylocaine. For the average-sized patient, a 3-inch 22-gauge spinal needle is inserted into the foramen. The operator needs to be aware of the sacral anatomy. The orientation of S4 to the skin surface is approximately 90 degrees, but it is only 60 degrees at S3 (
Fig. 12.1). The spinal needle probes the bone and then drops into the foramen, a movement that has a distinct feel. The insulated spinal needle is connected to the temporary stimulator (
Fig. 12.2). The parameters are preset at a stimulation frequency of 10 Hz, pulse duration 210 microseconds, and current of 0.5 to 20 milliamps. An appropriate S3 response is plantar flexion of the ipsilateral great toe and contraction of the levator ani, causing deepening of the groove between the buttocks, the so-called bellows response (
11). Toe flexion is not requisite. S4 stimulation results in appropriate sensation for the patient but no motor response. The patient typically reports a pulling sensation in the vagina and/or rectum or a vibrating or tingling sensation in the vagina or rectum. An S2 response would involve plantar flexion and an eversion of the foot and a withdrawal-type “clamp” of the rectum. This is not desirable.
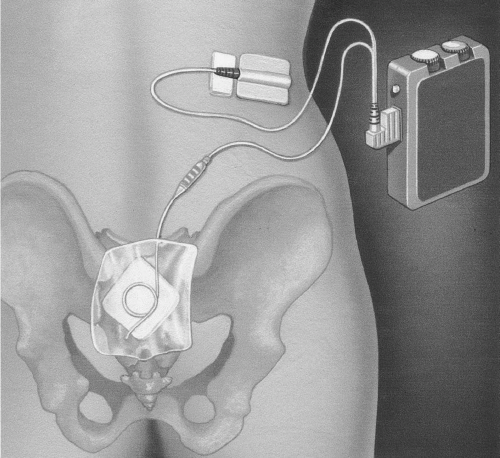
When an appropriate response is obtained, a 3057 PNE test electrode wire is threaded through the sheath of the spinal needle. It is important for the patient to avoid bending, as this may dislodge the wire. The lead is attached to the temporary stimulation device (
Fig. 12.3). The patient adjusts the voltage of the stimulation by turning a simple dial. She should feel the response at all times, but it should be comfortable. The patient should be made aware that the intensity may vary and may have to be adjusted accordingly depending on her position and activity. Direct comparison is made between a 3-day diary done during the PNE and the one performed before stimulation to determine if there has been at least a 50% improvement in symptoms to qualify for permanent implantation. Successful response for the test stimulation phase is reported at a mean of 55% (range 28% to 83%) (
Table 12.1) (
6,
12,
13,
14,
15,
16,
17,
18,
19,
20).
Incorrect placement is a reason for failure of test phase. Janknegt et al surgically implanted 10 nonresponders and noted that 8 obtained a positive response, indicating inappropriate positioning was the reason for failure (
21). Benson reported on the addition of electrodiagnosis (
22). A ring electrode located on a Foley catheter is placed in the urethra. The muscle response from the urethral sphincter is recorded. He reported a positive response rate of 80%. Of the responders, 46% did not have a bellows response, 74% no toe response, and 46% no vaginal/sensation response, the responses typically looked for when relying on perineal and extremity visualization.
Because of the number of failures and lead migration during the test phase resulting in fewer numbers of patients being eligible for the permanent implantation, the technique changed in 2003. Currently, the actual implantable electrode is placed and used for the test phase—the so-called staged implant.
Staged Implant
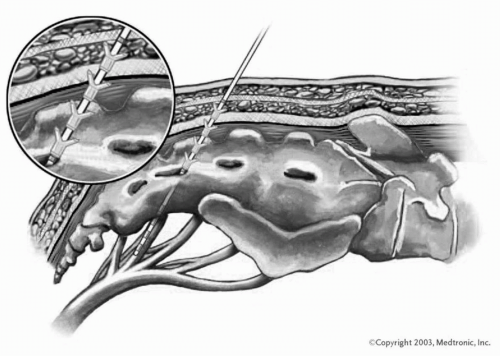
The initial test phase is now done in the operating room, at which time the tined lead is placed (
Fig. 12.4). This first stage typically runs between 1 and 4 weeks, with most physicians doing a patient trial for a minimum of 2 weeks. The time may be particularly important in patients with interstitial cystitis, as their condition will often wax and wane, and it may take an extended period of time to determine the effectiveness of the therapy.
There is no evidence that bilateral lead placement is advantageous (see later discussion), so most physicians will place one lead into the sacral foramina that appears to have the best motor response at the time of initial placement. The patient is placed in an identical position as in the older PNE phase. It is very important that the anesthesiologist be informed to avoid long-acting muscle relaxants, as these may interfere with the elicitation of an electromyographic (EMG) response from the anal sphincter/toe/foot. The patient must be adequately supported with chest rolls, arm supports, and pillows under the knees to avoid nerve injury and pressure necrosis. The feet are left exposed to determine the toe or foot response as deemed appropriate. The buttocks are taped apart so that the anal sphincter is readily apparent.
Many physicians initially place the spinal needle under fluoroscopy; however, for those not attuned to using fluoroscopy, it can sometimes be
difficult to determine the exact positioning, and the bony landmarks are still very useful. The position of the sciatic notch and the drop-off of the coccyx are the most consistent landmarks. For the patient who has a larger body mass index (BMI), the drop-off coccyx may be easier to palpate than the notch. It is important for the individual physician to determine how many fingerbreadths above the drop-off the S3 foramen is located (approximately three to four). The midline is marked and then the approximate location of S3 is 1.5 fingerbreadths lateral to the midline. A spinal needle similar to the old test stimulation needle is then placed at a 60-degree angle and advanced into S3. Attachment to the test stimulator confirms placement, as evidenced by contraction of the anal sphincter with elevation of the pelvis with or without plantar flexion of the great toe. If there is any doubt as to whether it is an S3 response, needles can be placed above or below the initial foramina to determine an S2 response. Fluoroscopy is often useful at this time, especially the lateral view to determine how far up on the sacrum the spinal needles are located.
After confirmation of an S3 response, a wire is then placed down the spinal needle into the foramina. A small incision is placed from the spinal needle down 0.5 cm to allow for easier placement of the plastic introducer. The rest of the placement is very similar to placing a central line (i.e., the use of the Saldinger technique). Once the wire is through the foramina, the spinal needle is removed. A wider introducer with a plastic covering is then inserted over the wire and through the foramina. There is a distinctive “pop” or give as this goes through the foramina. Fluoroscopy confirms that the plastic sheath is deep enough in the foramina. There is a small radiopaque ring at the tip of the sheath, and this should be just beyond the bony plate.
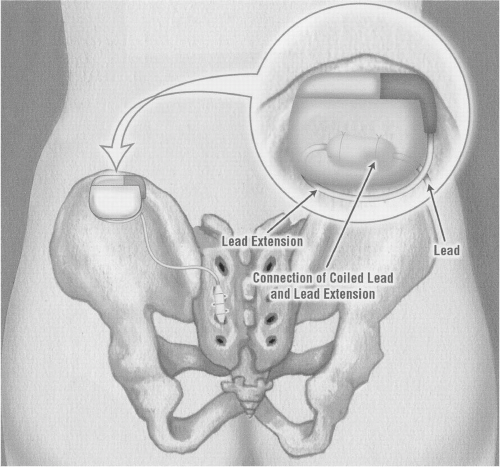
With a twist of the needle, the inside metal introducer is removed, leaving the plastic sheath in place. Through this plastic sheath, a tined lead is passed (
Fig. 12.5). The lead has four electrodes similar to the previous one, with the exception that electrode 1 is now wider to compensate for small degrees of lead movement that may occur postoperatively. The lead is passed down through the plastic sheath to the level of the first mark on the lead. This ensures that the electrodes are beyond the end of the plastic sheath but that the barbs of the tined lead are still within the plastic sleeve and not activated.
Progressive stimulation of the four individual electrodes is then performed to determine the optimal position of the lead. The lead can be adjusted deeper or more superficially by gently moving the plastic outer sheath and lead together. It is very important not to move the lead itself; otherwise, the tines may be activated prematurely and the lead cannot be readjusted after that. Ideally, at least two electrodes should give an EMG response. It is preferable to obtain a good motor response from electrode 1, as this is the widest electrode. If anything, the leads tend to be pulled back, so it is preferable to obtain a response from the more superficial leads 3, 2, and 1 as opposed to 0 and 1. This means that in the event that the lead is pulled back slightly, response may be lost in electrodes 2 or 3 but allows for 1 and 0 to pick up.
When ideal placement is confirmed, a lateral film is taken. This film is then left on the one side of the fluoroscopy viewer and under live view; an attempt is made to remove the outer plastic sheath while leaving the electrode configuration in the same position as the initial film. The lead is then tunneled to the buttock on the side selected for the programmer (IPG). A small pocket is fashioned to accommodate the interconnection piece. A special tunneling device is provided with the kit that enables smooth passage. A boot is placed over this wire, the interconnection lead attached to the electrode, and the boot secured in place with suture to minimize the risk of any fluid leaking into the connection area. The tunneling device is used again to tunnel from the buttock incision to the opposite side where the temporary lead exits. It is important to place the exit site as far away as possible from the future site of the IPG and the lead to minimize the risk of infection.
The operative sites are irrigated with sterile water. The incisions are closed. The buttock incision is typically closed in two layers to minimize the risk of seroma or hematoma, which increases the risk of infection. Sterile dressings are then applied and a large bio-occlusive dressing is placed over the whole area. Patients are typically covered with a broad spectrum of antibiotic for several days to a week after the procedure. The exteriorized wire is then connected to the patient programmer. The handheld temporary programmer is then set to the electrode configuration that was felt to be the optimal response in the operating room. The patient is then allowed to adjust the intensity of stimulation. The temporary programmer does allow for reprogramming between the different electrodes should an optimal response not be obtained initially. Success rates for this test stimulation have typically been higher than for the original PNE (see
Table 12.1) (
23,
24).