Henry Krum, Markus P. Schlaich, Paul A. Sobotka
Interventional Treatments for Resistant Hypertension
This chapter discusses novel procedure-based and device-based strategies in the management of systemic hypertension refractory to conventional treatment. Two of the most promising approaches are percutaneous renal sympathetic denervation and baroreceptor activation therapy.
Pathogenesis
Sympathetic Nervous System in Hypertension and Cardiovascular Disease
The contribution of sympathetic activation to the genesis and progression of systemic hypertension and cardiovascular disease (CVD) has been well recognized for many decades.1 A stepwise increase in muscle sympathetic nerve activity (MSNA) occurs from normal blood pressure (BP), high-normal BP, white coat hypertension, borderline hypertension, and established hypertension, with or without left ventricular hypertrophy (LVH).2 These data are supported by other measures of sympathetic activation in humans, specifically spillover of noradrenaline into plasma. In a study of renal spillover from the kidneys, a significant increase was noted in patients with primary hypertension compared with normotensive controls.3 This increase was particularly prominent in younger hypertensive participants (20 to 39 years).
Renal Efferent Sympathetic Activity
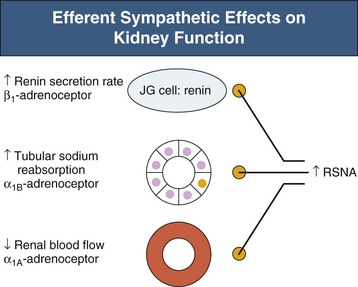
Renal sympathetic efferent nerves modulate autonomic control of the kidney. Renin secretion is activated by β1-adrenoceptor stimulation, enhanced tubular sodium reabsorption by α1B-adrenoceptors and reduced renal blood flow by α1A-adrenoceptors4 (Fig. 38-1). Thus, sympathetic innervation is critical to renal control of regulatory hormones, modulation of total body volume status, and effects on the pressure-natriuresis curve. Renal sympathetic denervation shifts the diuresis and natriuresis curves to the left5; that is, an increase in water and sodium excretion for the same renal perfusion pressure is achieved in the denervated compared to the innervated animal.
Abrogation or disruption of renal sympathetic efferents therefore represents an attractive therapeutic target in the management of disorders characterized by renal sympathetic nerve activation. This has been supported through the preclinical literature in both low-renin and high-renin models of hypertension in animals.6
Renal Afferent Sympathetic Activity
The kidney and pelvic region is highly innervated by mechanosensitive and chemosensitive nerve receptors.7 Renal afferent nerves transmit this information to the central sympathetic nervous system, which in turn modulates activity of key organs, including heart, kidney, and vasculature (Fig. 38-2). Indeed, dorsal rhizotomy (afferent denervation) reduces BP in animals with renal disease8; the renal afferent denervation abrogates increased CNS catecholamine levels. In renal transplant patients, denervation through nephrectomy of the nonfunctioning kidney reduced both renal sympathetic efferent activity and BP.9 Similarly, in patients with end-stage renal disease (ESRD) on dialysis, removal of the nonfunctioning kidney has simultaneously reduced MSNA and calf vascular resistance, confirming the kidney as a seminal source of sympathetic stimulation.10
Carotid Baroreflex Sensitivity
Abnormalities of the baroreflex are well described in the setting of systemic hypertension.11 Arterial baroreceptors are rapidly reset in response to sustained BP elevation but also buffer short-term fluctuations in BP.11 As BP increases, firing of baroreceptor afferents also increases. If BP elevation is sustained despite this, however, the baroreceptor response diminishes over time, and a new threshold for activation is established. Thus, baroreceptors become less sensitive to any given change in BP in patients with chronic hypertension. The reasons for this baroreceptor resetting are not well understood. This contribution of reduced baroreflex activity to chronic hypertension and its associated multiorgan involvement has been widely described.
Surgical Sympathetic Denervation
In the era preceding the emergence of modern antihypertensive pharmacotherapy, surgical denervation of the renal arteries was perhaps the only effective approach to treating patients with significant elevations in BP. Case series comparing this surgical approach to medical therapies (such as existed) demonstrated about 50% improvement in survival with denervation in patients for the same starting BP values.12 The magnitude of the BP reduction correlated with the starting preoperative mean BP. However, sympathetic denervation was accompanied by significant adverse events, limiting the clinical utility. In particular, patients experienced impotence, incontinence, and almost invariably, orthostatic hypotension, essentially rendering them unable to maintain upright posture for long periods.13
Percutaneous and Minimally Invasive Approaches to Renal Sympathetic Denervation
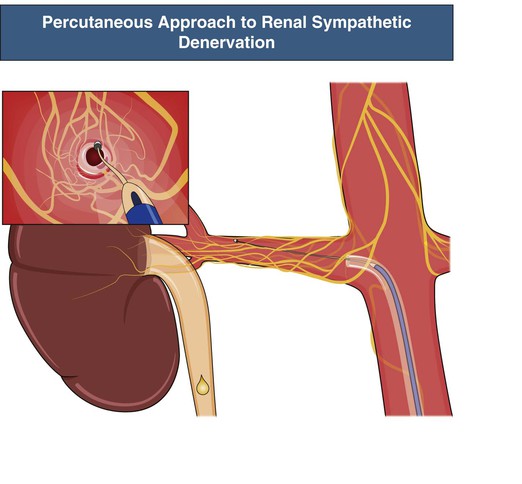
Novel approaches have been developed to achieve specific renal sympathetic denervation while avoiding the complications of the earlier surgical approaches, as outlined earlier. Most of these approaches focus on the sympathetic nerve plexus that surrounds the main trunk of each renal artery. These nerves reside within the adventitia of the main artery (or immediately adjacent). These novel methods include various approaches to radiofrequency (RF) energy application, use of ultrasound waves, direct injection of neurotoxins such as guanethidine or ethanol, and even extracorporeal approaches that are completely noninvasive. By far the most advanced and best investigated of these strategies is percutaneous RF ablation14 (see Fig. 38-2). This procedure involves cannulation of the femoral artery and subsequent placement of the catheter tip in the distal renal artery, where energy is applied to target adjacent sympathetic nerve trunks. The catheter is then withdrawn 1 to 2 cm and circumferentially rotated with further RF energy applications performed, such that four to six applications on average (often more) are applied to the individual renal artery. The same procedure is applied in both main renal arteries.
Published Experience with Percutaneous Renal Sympathetic Denervation
Symplicity Hypertension 1 Study
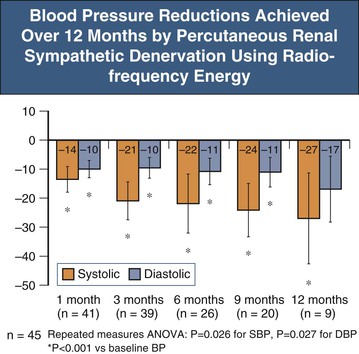
Symplicity HTN-1 was a 12-month evaluation of safety and BP-lowering efficacy (without a control group) as a first-in-human experience with the denervation procedure.15 Inclusion criteria involved patients with a systolic BP greater than 160 mm Hg despite three or more antihypertensive medications, including a diuretic or confirmed intolerance to medications. Furthermore, estimated glomerular filtration rate (eGFR) was required to be greater than 45 ml/min/1.73 m2. The key exclusion criteria included known secondary causes of hypertension, type 1 diabetes mellitus, central sympatholytic drug use, and evidence of renovascular abnormalities, including renal artery stenosis, prior renal procedure, and dual renal arteries. Enrolled patients had a mean BP greater than 170 mm Hg systolic and 100 mm Hg diastolic, despite taking on average five antihypertensive drug classes. Almost all patients were taking ACE inhibitor and/or angiotensin receptor blocker (ARB) as well as diuretics, and 75% were taking β-blockers. After 12 months, BP was reduced by 27/17 mm Hg compared to baseline (Fig. 38-3). Limited ambulatory blood pressure monitoring (ABPM) data showed an increase in patients shifting from “nondipper” to dipper status with the procedure. However, the magnitude of the ABPM response to denervation in this study (and Symplicity HTN-2) was substantially less than that of office BP decreases, suggesting a “white coat” component may be contributory to the observed office response.
The key mechanistic question in Symplicity HTN-1 was whether sympathetic denervation of the kidney is achieved by the renal denervation (RDN) procedure. In humans, evidence of renal denervation was observed with a substantial reduction in renal norepinephrine spillover rate in a case study in which BP decreased. In these patients, MSNA (indicative of efferent sympathetic output) also progressively decreased out to 12 months.16 Sympathetic nerve activity reduction after RDN has been confirmed in a larger series.17 However, it remains uncertain at the time of the procedure whether denervation has been successfully achieved. The extent of efferent and afferent denervation remains unquantifiable and the importance of complete denervation uncertain; there are as yet no simple clinical tools to address this question.
The Symplicity HTN-1 experience has been extended to 36 months in a larger cohort.18 Of the 153 patients followed, however, only 24 actually reached the 36-month follow-up visit. Nonetheless, the mean reduction in clinic BP persisted, with a mean 33/19 mm Hg reduction compared to baseline at 36 months. This is consistent with the surgical experience in which many years of improved BP control are observed after the surgical intervention. There is no evidence of loss of BP-lowering effect during the 36 months.
In this cohort, “responders” were arbitrarily defined as those with an office systolic BP reduction greater than 10 mm Hg versus baseline.18 At 12 months, only 79% had achieved this response in the Symplicity HTN-1 expanded cohort. By 36 months, however, all study patients had achieved this “response.” This raises the important issue of what physiologic mechanisms may occur after denervation to produce a late (but not early) BP effect. Possibilities include progressive vascular remodeling, resetting of the baroreflex, and alterations in renal blood flow and sodium excretory status, all of which may require time to “reset.” Whether different energy or ablation modalities could alter the responder rate or the time course of BP reduction remains unknown.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree