Issues in Intervascular Procedures
Over the past decade, there has been a resurgence of interest by nephrologists in the management of hemodialysis vascular access. The early days of dialysis were marked by advances in vascular access conceived and developed by visionary nephrologists, including the Scribner shunt and the Brescia-Cimino arteriovenous (AV) fistula. Without these means of obtaining reliable repeated blood access, the delivery of chronic hemodialysis would not have been possible. Some nephrologists have maintained this primary role in the creation and maintenance of vascular access, particularly in Europe. One successful example reported the construction of a series of 748 consecutive native AV fistulas, with 2 year assisted access survival rates in diabetics and nondiabetics ranging from 75% to 96%. During the 1970s and 1980s, at least in the United States, interest and involvement in vascular access largely faded. This may have been due to exciting progress in what were perceived to be more scientifically rewarding areas of study, as opposed to the relatively mundane “plumbing” problems of vascular access. Certainly neither technical proficiency nor rigorous academic attention to vascular access was emphasized in most nephrology training centers in the United States during that time. In many programs and practices management of vascular access was left exclusively to the surgeons. At the same time, particularly in the United States, there was increased promotion and utilization of synthetic polytetrafluoroethylene (PTFE) grafts in favor of native AV fistulas. This shift may have been driven by marketing and reimbursement practices, poor long-term venous access catheters available for use as “bridges” to native fistulae, and increasing emphasis on short, high efficiency dialysis treatments. The result for the United States nephrology community was a large hemodialysis patient population with a high prevalence of PTFE grafts, a low usage of AV fistulas, and perhaps incidentally, the highest dialysis patient mortality of all industrialized nations. In 1999, 49% of hemodialysis patients in the United States were dialyzing with AV grafts, 28% with native fistulas, and 23% with venous catheters.
During this period of a rapidly growing hemodialysis patient population, increasing PTFE graft utilization, and decreased involvement of nephrologists in the management of vascular access problems, there was a predictable crisis in the access-related medical care of these patients. Management of access dysfunction and thrombosis was largely “reactive” and primarily utilized open surgical techniques. The role of venous stenosis in contributing to AV graft thrombosis and failure was underappreciated. In the late 1980s, interventional radiologists began to recognize these problems and applied their tools and techniques to treating access dysfunction. A method for declotting AV hemodialysis grafts using pharmacomechanical thrombolysis and angioplasty was reported in 1991. Numerous other reports and variations on this method followed, with increasing acceptance of percutaneous interventions in the management of hemodialysis access dysfunction. Largely, however, nephrologists remained on the periphery, as vascular access continued to be the province of the vascular surgeon and more recently interventional radiologists. This collaboration of expert subspecialties might have been all that was needed to provide timely, high-quality hemodialysis access care. Undeniably, in some settings, this was the case. However, while access dysfunction was of critical and immediate importance to the patient, dialysis unit, and nephrologist, for many programs this could not be the first priority for the surgeons or radiologists, creating a service void and an opportunity for improvement in care.
The central role of vascular access in the care of patients on hemodialysis cannot be overemphasized. Comprehensive medical care of the hemodialysis patient includes management of uremia, hypertension, sodium and water balance, anemia, mineral metabolism, metabolic bone disease, and nutritional status. This care cannot be properly delivered unless there is reliable, efficient blood access for dialysis. Leaving this critical aspect of care entirely in the hands of others puts the patient and the nephrologist at a significant disadvantage. Under ideal circumstances, when the skills and priorities of the multispecialty access team come together, patient care may be very well served. Conversely, if the appropriate surgical or interventional services cannot be delivered in a timely fashion, the patient may suffer in terms of delayed dialysis, temporary venous hemodialysis access, unnecessary hospitalization, or other avoidable morbidity. This also may result in a significantly greater financial burden to the health care system.
In the early 1990s, this problem was recognized and confronted by Dr. Gerald Beathard in Austin, Texas. He acquired the necessary training, adapted the reported interventional radiology techniques, and developed a nephrology-run service for percutaneous management of vascular access. He then liberally shared this expertise, training many nephrologists from various practices and backgrounds, this author included. It is a remarkable fact that all interventional nephrologists practicing in the United States can trace their roots directly back to Dr. Beathard. As these nephrologists brought these techniques to their practices, the field of “interventional nephrology” was effectively born. When nephrologists began to perform these access-related percutaneous interventions, the first priorities were to master the techniques, establish suitable facilities in which to work, and then deal with the multitude of day-to-day access failures, largely centered on the PTFE graft. While this led to an immediate and dramatic improvement in care, it was very evident that the poor performance of PTFE grafts compared to native AV fistulas was contributing to an excessively high rate of access failure and hence a large volume of percutaneous interventions. This was good business, but very bad medicine. This realization led to the next phase in the evolution of interventional nephrology, which was to take on comprehensive vascular access management for the patient on hemodialysis. To improve vascular access outcomes, it would be folly to address only the technical aspects of percutaneous interventions. To achieve optimal vascular access, the following aspects of care take on equal or greater importance.
- Preservation of peripheral veins for native AV fistulas.
- Avoidance of peripherally inserted central venous catheters and subclavian catheters.
- Judicious use of internal jugular vein tunneled hemodialysis catheters for temporary hemodialysis access.
- Early referral to a surgeon for construction of a native AV fistula.
- Education and selection of surgical colleagues willing and able to master the techniques for the creation of a native AV fistula.
- Preoperative imaging of veins and arteries, including ultrasound vein mapping and venography.
- Evaluation and treatment of a poorly functioning or immature AV fistula.
- Conversion from a failing PTFE graft to a “secondary” native AV fistula.
- Maintenance of a complete and accurate clinical database with regular quality analysis including rates of usage of AV fistulas and catheters success rates of technical procedures, complications, and patient satisfaction.
Another essential principle must be recognized in order to provide optimal hemodialysis access care: For each scenario of access dysfunction, there is a “best solution.” There has been a tendency for vascular access solutions to follow “the path of least resistance” or, worse yet, “the path of greatest reimbursement,” both of which may be very different from the optimal pathway. From the perspective of the interventionist, there is the temptation to view all problems as best solved using percutaneous means. However, there are clearly situations in which surgical solutions are preferable and should be employed. This of course works both ways. It would be equally poor care to perform frequent, repeated percutaneous thrombectomies and angioplasties on a failing PTFE graft as it would be to attempt an open surgical declot of a fistula that thrombosed due to central venous stenosis. Knowing the anatomy and history of each patient is the critical element that allows these judgments to be made correctly. In this regard, keeping a detailed clinical database is essential. This allows the operator to fully assess the vascular access problem at hand, make the most appropriate management decision based upon the history and known anatomic factors, and perform the necessary procedure with the lowest risk and best possibility of a successful outcome.
When a patient presents with access dysfunction, a timely solution is required. This may represent an urgent problem such as a thrombosed AV fistula that requires immediate intervention to salvage the access and provide dialysis. Although arguably not a true “medical emergency,” restoration of fistula or graft function is of paramount importance to the care of the patient on hemodialysis, with potentially grave implications for both short-term and long-term morbidity and mortality. Other access problems may be less immediate, such as prolonged bleeding from needle puncture sites related to venous outflow stenosis; this may not prevent dialysis, but puts the patient at risk, may lead to access thrombosis, and should be dealt with before it becomes an urgent problem. Other access problems may be relatively elective in nature, such as the evaluation of limb swelling associated with central venous stenosis or a slowly enlarging pseudoaneurysm. In all cases, the goal of an interventional program should be to address each problem in an appropriate timely fashion, minimizing disruption of the dialysis schedule of patients. Nephrologists are in an ideal position to provide these services when equipped with the necessary skills, allowing for seamless delivery of care and management of dialysis-related problems as required before, during, and after an interventional procedure. Alternatively, there are many practices in which excellent care and service are provided by interventional radiologists or vascular surgeons. The title of the individual responsible for vascular access interventions is not as important as his or her knowledge, skill, availability, and willingness to work with surgeons, nephrologists, and the dialysis staff as part of a multidisciplinary access management team.
The American Society of Diagnostic and Interventional Nephrology (ASDIN) was founded in 2000 to promote the proper application of procedures in the practice of nephrology. These include diagnostic ultrasound imaging and peritoneal dialysis catheter placement in addition to the full complement of percutaneous interventions required for management of hemodialysis vascular access. A central goal of this society was to develop standards for training, certification, and accreditation relative to these diagnostic and interventional disciplines. These were published in 2003 and remain the only standards specific to these procedures. Previously there were no specific standards and there was tremendous variation in the training and credentialing requirements of health care facilities. It is expected that with increasing awareness and acceptance of these criteria in the United States and elsewhere, interventional training will become more uniform and rigorous, ultimately improving the quality of care offered as well as patient outcome. Over the past several years, interventional nephrology training programs have become established at some U.S. academic centers. As these centers develop, they should be expected to advance the standard for training, quality, and clinical research related to vascular access.
Core Endovascular Procedures
The core procedures of interventional nephrology include the placement and management of venous hemodialysis catheters, diagnostic imaging of AV accesses and native veins, percutaneous angioplasty, and percutaneous thrombectomy of occluded AV access. Other related procedures include the placement of venous stents, the ligation or embolization of native fistula accessory branches, and the implantation of subcutaneous venous hemodialysis access ports.
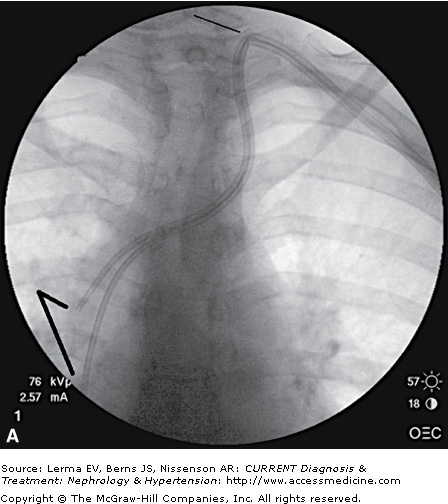
Venous catheters are the least desirable method of hemodialysis access. In a sense, every placement of a venous catheter represents a failure to prepare a native fistula in advance of initiating dialysis as well as a failure to detect a dysfunctional AV access and to intervene preemptively either to maintain its function or to create a new alternative access. Nevertheless, venous catheters are an unavoidable necessity for many patients who do not have functional AV access. For most interventional nephrologists, insertion of tunneled dialysis catheters is the first and most basic procedure acquired, building on the common skill of temporary hemodialysis catheter placement. Nevertheless, the risks of this procedure should not be understated, with the potential for severe injury to major central vessels from large-bore catheters and dilators. The use of real-time ultrasound guidance is widely considered to be essential for safe and efficient venipuncture based on higher procedural success rates and fewer complications compared to the use of landmarks only. The low posterior approach to the right internal jugular vein is ideal; this keeps the catheter low on the neck, with minimal patient discomfort and a good cosmetic result, and creates a smooth bend of the catheter that avoids kinking. Fluoroscopy is also recommended for proper catheter tip positioning, although there is limited evidence to support this requirement. There is general agreement that the performance of chronic dialysis catheters is optimized when the catheter tips are placed in the right atrium, and Disease Outcomes Quality Initiative (DOQI) guidelines support this approach. Nevertheless, this remains controversial, and achieving the desired tip position is difficult, even with fluoroscopy and careful attention to anatomic landmarks. In any case, to achieve the best possible outcomes, the operator must adhere to a meticulous sterile surgical technique, utilize ultrasound guidance for venipuncture, and pay careful attention to tip positioning using fluoroscopic and/or landmark guidance. Figure 57–1A shows a poorly placed left internal jugular vein tunneled dialysis catheter, with a high vein puncture from the anterior approach, a tight bend with the catheter kinked, and its split tips extending only into the superior vena cava. This catheter did not function for dialysis. Figure 57–1B shows the catheter replaced with a new Ash-Split catheter (Medcomp, Harleysville, PA), using the right internal jugular vein puncture from a low posterior approach with a smooth bend in the neck. In Figure 57–1C, the new catheter tips are shown extending into the high right atrium, yielding excellent catheter function as required for delivery of hemodialysis.