Infertility
Male reproductive physiology
Hypothalamic-pituitary-testicular axis
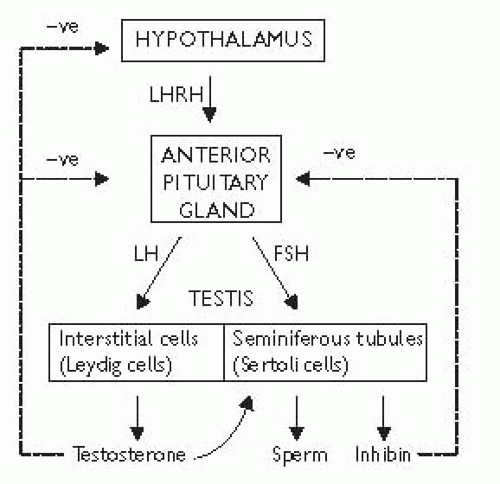
The hypothalamus secretes luteinizing hormone-releasing hormone (LHRH), also known asgonadotrophin-releasing hormone (GnRH). This causes the pulsatile release of anterior pituitary gonadotrophins called follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which act on the testis. FS H stimulates the seminiferous tubules to secrete inhibin and produce sperm; LH acts on Leydig cells to produce testosterone (Fig. 12.1).
Testosterone is secreted by the interstitial Leydig cells, which lie adjacent to the seminiferous tubules in the testis. It promotes the development of the male reproductive system and secondary sexual characteristics. Steroidogenesis is stimulated by a cAMP-protein kinase C mechanism which converts cholesterol to pregnenolone. Further steps in the biosynthesis pathway produce intermediary substances (dehydroepiandrosterone and androstenedione) priorto producing testosterone. In blood, 60% of testosterone is attached to sex hormone-binding globulin (SHBG), 38% bound to albumin and 2% is free. At androgen-responsive target tissues, testosterone is converted into a potent androgen, dihydrotestosterone (DHT), by intracellular 5α-reductase (see  p. 672; Fig. 16.7).
p. 672; Fig. 16.7).
 p. 672; Fig. 16.7).
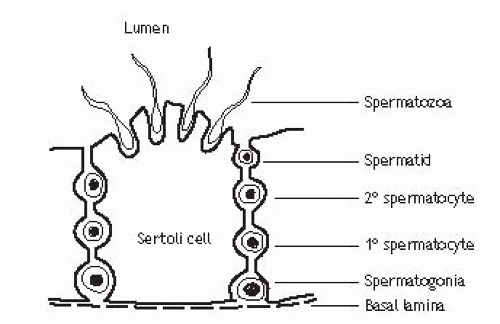
p. 672; Fig. 16.7).Spermatogenesis Seminiferous tubules are lined with Sertoli cells, which surround developing germ cells (spermatogonia) and provide nutrients and stimulating factors as well as secreting androgen-binding factor and inhibin (Fig. 12.2). Primordial germ cells divide to form primary spermatocytes. These undergo a first meiotic division to create secondary spermatocytes (46 chromosomes), followed by a second meiotic division to form spermatids (23 chromosomes). Finally, these differentiate into spermatozoa. This process takes 72 days. The non-motile spermatozoa leave the seminiferous tubules and pass to the epididymis for storage and maturation (until ejaculation). Spermatozoa that are not released are reabsorbed by phagocytosis.
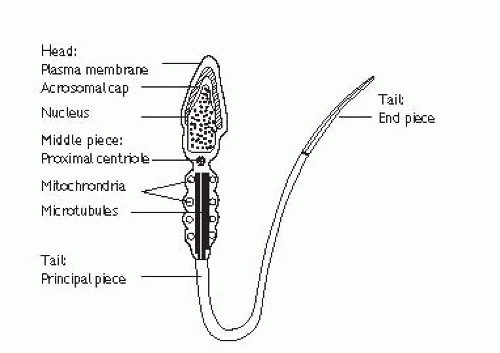
Mature sperm has a head, middle piece and tail (Fig. 12.3). The head is composed of a nucleus covered by an acrosome cap containing vesicles filled with lytic enzymes. The middle piece contains mitochondria and contractile filaments which extend into the tail to aid motility. After deposition at the cervix, sperm penetrate cervical mucus and travel through the uterus to the site of fertilization in the Fallopian tube, during which time they undergo functional maturation (capacitation). Sperm start to penetrate the oocyte and bind to the zona pellucida. The activation phase is initiated (by ZP3), triggering hyperactivated motility and the acrosomal reaction which leads to enzyme release, penetration into the cytoplasm of the oocyte, fusion, and fertilization.
Aetiology and evaluation of male infertility
Definition of subfertility
Failure of conception after at least 12 months of regular unprotected intercourse. The chance of a normal couple conceiving is estimated at 20-25% per month, 75% by 6 months, and 90% at 1y.
Epidemiology
Up to 50% of infertility is due to male factors. An estimated 14-25% of couples may be affected at some point in their reproductive years. Twenty-five percent of subfertile couples will have oligozoospermia and 10% azoospermia. Azoospermia exists in 1% of the male population.
Pathophysiology
Failed fertilization of the normal ovum due to defective sperm development, function, or inadequate numbers. There may be abnormalities of morphology (teratozoospermia, <4% normal forms), motility (asthenozoospermia, <40% motile sperm), low sperm numbers (oligozoospermia, <15 × 106 per mL), or absent sperm (azoospermia). Abnormal epididymal function may result in defective spermatozoa maturation or transport or induce cell death.
Aetiology of male factor infertility
Idiopathic (25%).
Varicocele (present in ~40%).
Undescended testes.
Functional sperm disorders: immunological infertility (antisperm antibodies); head or tail defects; Kartagener’s syndrome (immotile cilia); dyskinetic cilia syndrome.
Erectile dysfunction.
Ejaculatory problems: retrograde ejaculation causes absent or low volume ejaculate.
Testicular injury: orchitis (post-pubertal bilateral mumps orchitis); testicular torsion; trauma; radiotherapy.
Endocrine disorders: Kallmann’s syndrome (isolated gonadotrophin deficiency causing hypogonadism); Prader-Willi syndrome. (hypogonadism, short stature, hyperphagia, obesity); pituitary gland adenoma, radiation, or infection.
Hormone excess: excess prolactin (pituitary tumour); excess androgen (adrenal tumour, congenital adrenal hyperplasia, anabolic steroids); excess oestrogens.
Genetic disorders: including Klinefelter’s syndrome (47XXY) with azoospermia, ↑ FSH/LH and ↓ testosterone; XX male and XYY syndromes. Deletions in the azoospermic factor (AZF) gene on the Y chromosome are associated with abnormal spermatogenesis, which can be inherited by male offspring. Microdeletions of region AZFa has associations with Sertoli cell only syndrome; AZFb with maturation arrest and AZFc with azoospermia/severe oligozoospermia.
Male genital tract obstruction: congenital absence of vas deferens; agenesis of seminal vesicles/Wolffian duct abnormalities; epididymal obstruction or infection; Müllerian prostatic cysts; inguinoscrotal or pelvic surgery.
Systemic disease: renal failure; liver cirrhosis; cystic fibrosis.
Drugs: chemotherapy; steroids; alcohol; marijuana; sulphasalazine; smoking.
Environmental factors: pesticides; heavy metals; hot baths.
Infection: genital tract infections are found in 10-20%. Chlamydia trachomatis can attach to and penetrate sperm; Ureaplasma urealyticum reduces sperm motility. HIV infection, previous prostatitis, and bilateral epididymitis reduce semen quality.
History
Sexual and reproductive: duration of problem; frequency and timing of intercourse; use of vaginal lubricants (adversely affects sperm function); previous successful conceptions; previous birth control; erectile or ejaculatory dysfunction.
Partner’s history: age; previous pregnancies; previous investigation for subfertility; medical history.
Developmental: age at puberty; history of undescended testes; gynaecomastia.
Medical and surgical: detailed assessment for risk factors—recent febrile illness; post-pubertal mumps orchitis; varicocele; testicular torsion, orchidopexy, trauma or tumour; sexually transmitted infections; UTI; genitourinary and pelvic surgery; radiotherapy; respiratory diseases associated with ciliary dysfunction; diabetes.
Drug and environmental: previous chemotherapy; exposure to substances which impair spermatogenesis or erectile function; alcohol consumption; smoking habits; hot baths.
Family history: hypogonadism; undescended testes.
Examination
Perform a full assessment of all systems, with attention to general appearance (evidence of secondary sexual development; signs of hypogonadism; gynaecomastia). Urogenital examination should include assessment of the penis (phimosis, hypospadias, chordee); the presence of testes; measurement of testicular consistency, tenderness and volume with a Prader orchidometer (normal >20mL—varies with race); palpate epididymis (tenderness, swelling) and spermatic cord (vas deferens present or absent; varicocele); DRE of prostate.
Of note, the patient’s partner should also undergo full screening and assessment for infertility by a gynaecologist, in either a separate consultation or in a joint clinic.
Investigation of male infertility
Basic investigations
Semen analysis: 2-3 specimens over several weeks, collected after 2-7 days of sexual abstinence. Deliver specimens to the laboratory within 1h (ideally keeping the specimen warm in a shirt or trouser pocket). Ejaculate volume, liquefaction time, and pH are noted (Table 12.1). Microscopy techniques measure sperm concentration, total numbers, morphology, and motility (Table 12.2). The mixed agglutination reaction (MAR) test is used to detect antisperm antibodies (useful for asthenozoospermia which can be associated with immunological infertility). The presence of leucocytes (>1 × 106/mL in semen) suggests infection and cultures should be requested. Low or absent ejaculate volume may suggest abnormality of seminal vesicles, ejaculatory duct obstruction, hypogonadism, or retrograde ejaculation.
Hormone measurement: serum FSH, LH and testosterone (Table 12.3). In cases of isolated low testosterone level, it is recommended to test early morning and free testosterone levels. Raised prolactin is associated with sexual dysfunction and may indicate pituitary disease.
Special investigations
Karyotype: 5-10% of azoospermic patients have Klinefelter’s syndrome.
Y chromosome microdeletion assay: to assess AZF—regions a, b, and c.
AZFa: microdeletion predicts no spermatogenesis.
AZFc: commonest molecular cause of male infertility (13% of nonobstructive azoospermics and 6% of oligozoospermics). Around 70% will have sperm on testis biopsy.
Post-orgasmic urine analysis: the presence of >10-15 sperm per high powered field confirms the diagnosis of retrograde ejaculation.
Imaging
Scrotal USS: is used to assess for testicular abnormalities and detection of varicocele.
Transrectal USS: is indicated for low ejaculate volumes, to investigate seminal vesicle obstruction (>1.5cm width) or absence and ejaculatory duct obstruction (>2.3mm).
Vasography: the vas deferens is punctured at the level of the scrotum and injected with contrast. A normal test shows the passage of contrast along the vas deferens, seminal vesicles, ejaculatory duct, and into the bladder, which rules out obstruction.
Venography: used to diagnose and guide embolization treatment of varicocele.
Testicular biopsy
Performed for azoospermic patients to help differentiate between obstructive and non-obstructive causes. Simultaneous sperm retrieval can be carried out (testicular sperm extraction, TESE) for use in intracytoplasmic sperm injection (ICSI) treatment, either at the time or at a later date (following freezing and storage). The degree of spermatogenesis can be histologically scored (this is the Johnsen score; Table 12.4). Only mature spermatozoa (score 8 or above) can be used for fertility treatment.
Table 12.1 WHO semen analysis characteristics | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree