Urological Investigations
Assessing kidney function
When we talk about measuring kidney function, what we mean is measurement of glomerular filtration rate (GFR). This is regarded as the best measure of kidney function and we grade the degree of renal impairment and renal failure according to the GFR. Normal GFR in young men is approximately 130mL/min per 1.73m2 of body surface area. In young women, it is 120mL/min per 1.73m2 of body surface area. Mean GFR declines with age (Table 3.1).
The ideal filtration marker is excreted by filtration alone. Exogenous markers that can be used to measure include inulin, iothalamate, ethylene diamine tetra-acetic acid (EDTA), diethylene triamine penta-acetic acid, and iohexol. Measurement of GFR using exogenously administered markers is complex and expensive and is difficult to do in routine clinical practice.
Urinary clearance of endogenous markers, such as creatinine, can be used to estimate GFR. Creatinine is a 113D-amino acid derivative that is freely filtered at the glomerulus. A timed urine collection and measurement of serum creatinine concentration allows calculation of GFR according to the formula:
where U is the concentration of urine in urine, P the concentration in plasma, and V the urine flow.
As an alternative, estimation of GFR can be made from simple measurement of serum creatinine since the main mechanism of creatinine excretion is by glomerular filtration and GFR has a reciprocal relationship with serum creatinine. Thus, as GFR falls (indicating worsening renal function), creatinine rises. However, creatinine is not the ideal filtration marker since it is also excreted by proximal tubular secretion as well as by glomerular filtration and therefore, creatinine clearance exceeds GFR, i.e. creatinine clearance tends to overestimate GFR.
Estimated GFR (eGFR)
Since the endogenous production of creatinine is determined by muscle mass, serum levels of creatinine will not only vary according to renal function (glomerular filtration), but also according to age, body size, ethnic group, and sex. Taking account of these factors can overcome some of the limitations of measurement of serum creatinine alone.
Two equations have been widely used for calculating eGFR—the Cockcroft-Gault formula and the Modification of Diet in Renal Disease (MDRD) equation. Both were developed from populations of patients with chronic kidney disease. They are less accurate estimates of renal function in populations without chronic kidney disease.
The Cockcroft-Gault formula (overestimates GFR because of tubular secretion of creatinine and the value is not adjusted for body surface area).
The MDRD equation (modified in 2005; adjusts for body surface area).
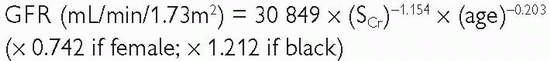
The MDRD is reasonably accurate as an estimate of GFR, the mean difference between eGFR and measured GFR ranging from -5 to 1mL/min/1.73m2.
The eGFR provides substantial improvements over serum creatinine measurements alone in the clinical assessment of renal function in terms of the detection, evaluation, and management of chronic kidney disease (Table 3.1).
Urine examination
Dipstick testing
Analysis for pH, blood, protein, glucose, and white cells can be done with dipstick testing.
pH
Urinary pH varies between 4.5 and 8, averaging between 5.5 and 6.5.
Blood
Normal urine contains <3 RBCs per high-powered field (HPF) (1000 erythrocytes/mL of urine; upper limit of 5000-8000 erythrocytes/mL). Positive dipstick for blood indicates the presence of haemoglobin in the urine. Haemoglobin has a peroxidase-like activity and causes oxidation of a chromogen indicator which changes colour when oxidized. Sensitivity of urine dipsticks for identifying haematuria (>3 RBCs/HPF is >90%); specificity is lower (i.e. a higher false positive rate with the dipstick due to contamination with menstrual blood, dehydration (concentrates what RBCs are normally present in urine)).
Haematuria due to a urological cause does not elevate urinary protein. Haematuria of nephrological origin often occurs in association with casts and there is almost always significant proteinuria.
Protein
Normal, healthy adults excrete about 80-150mg of protein per day in their urine (normal protein concentration <20mg/dL). Proteinuria suggests the presence of renal disease (glomerular, tubulo-interstitial, renal vascular) or multiple myeloma, but it can occur following strenuous exercise. Dipstick test is based on a tetrabromophenol blue dye colour change (green colour develops in the presence of protein of >20mg/dL).
White blood cells
Leukocyte esterase activity detects the presence of white blood cells in the urine. Leukocyte esterase is produced by neutrophils and causes a colour change in a chromogen salt on the dipstick. Not all patients with bacteriuria have significant pyuria. False negatives: concentrated urine, glycosuria, presence of urobilinogen, consumption of large amounts of ascorbic acid. False positives: contamination.
Nitrite testing
Nitrites in the urine suggest the possibility of bacteriuria. They are not normally found in the urine. Many species of Gram negative bacteria can convert nitrates to nitrites and these are detected in urine by a reaction with the reagents on the dipstick, which form a red azo dye. The specificity of the nitrite dipstick for detecting bacteriuria is >90% (false positive nitrite testing is contamination). Sensitivity is 35-85% (i.e. lots of false negatives); less accurate in urine containing fewer than 105 organisms/mL.
Cloudy urine that is positive for white blood cells and nitrite-positive is very likely to be infected.
Urine microscopy
Red blood cell morphology
Determined by phase contrast microscopy. RBCs derived from the glomerulus are dysmorphic (they have been distorted by their passage through the glomerulus). RBCs derived from tubular bleeding (tubulointerstitial disease) and those from lower down the urinary tract (i.e. urological bleeding from the renal pelvis, ureters, or bladder) have a normal shape. Glomerular bleeding is suggested by the presence of dysmorphic RBCs, RBC casts, and proteinuria.
Casts
A protein coagulum (principally, Tamm-Horsfall mucoprotein derived from tubular epithelial cells) formed in the renal tubule and ‘cast’ in the shape of the tubule (i.e. long and thin). The protein matrix traps tubular luminal contents. If the cast contains only mucoproteins, it is called a hyaline cast. Seen after exercise, heat exposure, and in pyelonephritis or chronic renal disease. RBC casts contain trapped erythrocytes and are diagnostic of glomerular bleeding, most often due to glomerulonephritis. White blood cell casts are seen in acute glomerulonephritis, acute pyelonephritis, and acute tubulointerstitial nephritis.
Crystals
Specific crystal types may be seen in urine and help diagnose underlying problems (e.g. cystine crystals establish the diagnosis of cystinuria). Calcium oxalate, uric acid, and cystine are precipitated in acidic urine. Crystals precipitated in alkaline urine include calcium phosphate and triple phosphate (struvite).
Urine cytology
Urine collection for cytology: exfoliated cells lying in urine that has been in the bladder for several hours (e.g. early morning specimens) or in a urine specimen that has been allowed to stand for several hours are degenerate. Such urine specimens are not suitable for cytological interpretation. Cytological examination can be performed on bladder washings (using normal saline) obtained from the bladder at cystoscopy (or following catheterization) or from the ureter (via a ureteric catheter or ureteroscope). The urine is centrifuged and the specimen obtained is fixed in alcohol and stained by the Papanicolaou technique.
Normal urothelial cells are shed into the urine and under the microscope, their nuclei appear regular and monomorphic (diffuse, fine chromatin pattern, single nucleolus).
Causes of a positive cytology report (i.e. abnormal urothelial cells seen—high nuclear:cytoplasmic ratio, hyperchromatic nuclei, prominent nucleoli):
Previous radiotherapy (especially if within the last 12 months).
Previous cytotoxic drug treatment (especially if within the last 12 months, e.g. cyclophosphamide, busulfan, ciclosporin).
Urinary tract stones.
Renal adenocarcinoma (clear cell cancer of the kidney) usually does not exfoliate abnormal cells, although occasionally clusters of clear cells may be seen, suggesting the diagnosis.
High-grade urothelial cancer and carcinoma in situ exfoliate cells which look very abnormal and usually the cytologist is able to indicate that there is a high likelihood of a malignancy. Low-grade bladder TCC exfoliates cells which look very much like normal urothelial cells. The difficulty arises where the cells look abnormal, but not that abnormal—here, the likelihood that the cause of the abnormal cytology is a benign process is greater.
Sensitivity and specificity of positive urine cytology for detecting TCC of the bladder depends on the definition of “positive’—if only obviously malignant or highly suspicious samples are considered positive, then the specificity will be high. Urine cytology may be negative in as many as 20% of high-grade cancers. If “atypical cells’ are included in the definition of ‘abnormal’, the specificity of urine cytology for diagnosing urothelial cancer will be relatively poor (relatively high number of false positives) because many cases will have a benign cause (stones, inflammation).
Prostatic-specific antigen (PSA)
(see also  pp. 318-321)
pp. 318-321)
 pp. 318-321)
pp. 318-321)PSA is a 34kD glycoprotein enzyme produced by the columnar acinar and ductal prostatic epithelial cells. It is a member of the human kallikrein family and its function is to liquefy the ejaculate, enabling fertilization. PSA is present in both benign and malignant cells, although the expression of PSA tends to be reduced in malignant cells and may be absent in poorly differentiated tumours. Large amounts are secreted into the semen and small quantities are found in the urine and blood.
The function of serum PSA is unclear, although it is known to liberate the insulin-like growth factor type 1 from one of its binding proteins. Seventy-five percent of circulating PSA is bound to plasma proteins (complexed PSA) and metabolized in the liver, while 25% is free and excreted in the urine. Complexed PSA is stable, bound to alpha-1 antichymotrypsin and alpha-2 macroglobulin. Free PSA is unstable, recently found to consist of two isoforms:pro-PSA is a peripheral zone precursor, apparently elevated in the presence of prostate cancer, and BPSA is the transition zone precursor and associated with BPH. The half-life of serum PSA is 2.2 days. The normal range for the serum PSA assay in men is <4.0ng/mL, though this varies with age. Table 3.2 shows a published age-specific normal range (95th centile).
In the absence of prostate cancer, serum PSA concentrations also vary physiologically, according to race and prostate volume.
Radiological imaging of the urinary tract
Ultrasound
A non-invasive method of urinary tract imaging. While it provides good images of the kidneys and bladder, anatomical detail of the ureter is poor and the mid-ureter cannot be imaged at all by ultrasound because of overlying bowel gas.
Uses of ultrasound
Renal
Assessment of haematuria.
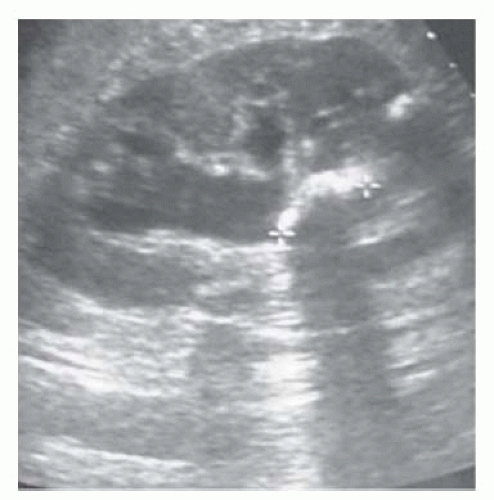
Determination of nature of renal masses—can differentiate simple cysts (smooth, well-demarcated wall, reflecting no echoes; benign) from solid masses (almost always malignant; cystic masses with solid components or multiple septae or calcification may be malignant), from those casting an ‘acoustic shadow’ (stones; Fig. 3.1).
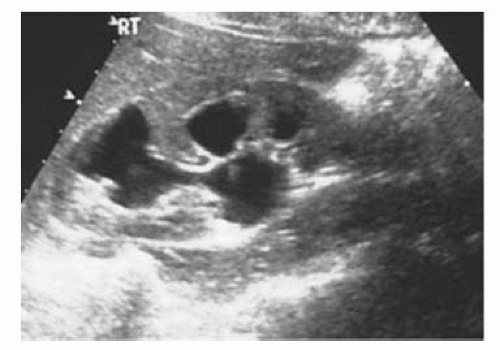
Can determine the presence/absence of hydronephrosis (dilatation of the collecting system) in patients with abnormal renal function (Fig. 3.2).
Allows ultrasound-guided nephrostomy insertion in patients with hydro-nephrosis and renal impairment or with infected, obstructed kidneys.
Bladder
Measurement of post-void residual urine volume.
Allows ultrasound-guided placement of a suprapubic catheter.
Prostate: TRUS
Measurement of prostate size (where gross prostatic enlargement is suspected on the basis of a DRE and surgery in the form of open prostatectomy is contemplated).
To assist prostate biopsy (allows biopsy of hypo- or hyper-echoic lesions).
Investigation of azoospermia (can establish the presence of ejaculatory duct obstruction).
Urethra
Can image the urethra and establish the depth and extent of spongiofibrosis in urethral stricture disease.
Testes
Assessment of the patient complaining of a ‘lump in the testicle (or scrotum)’—can differentiate benign lesions (hydrocele, epididymal cyst) from malignant testicular tumours (solid, echo poor, or with abnormal echo pattern).
When combined with power Doppler, can establish the presence/absence of testicular blood flow in suspected torsion.
Assessment of testicular trauma (rupture is indicated by abnormal echo pattern due to blood within the body of the testis; surrounding haematoma may be seen—blood within the scrotal soft tissues that has escaped through a tear in the tunica albuginea and the visceral and
parietal layers of the tunica vaginalis; haematocele—blood contained by an intact parietal layer of the tunica vaginalis).
Investigation of infertility—varicoceles and testicular atrophy may be identified.
Uses of plain abdominal radiography (the ‘KUB’ X-ray—kidneys, ureters, bladder)
For detection of stones and determination of their size and (to an extent) their location within the kidneys, ureters, and bladder (Fig. 3.3).
Renal calculi: a calcification overlying the kidneys is intrarenal if it maintains its relationship to the kidney on inspiratory and expiratory films (i.e. if it moves with the kidney). If in doubt as to whether an opacity overlying the outline of the kidney is intrarenal or not, get an ultrasound (look for the characteristic ‘acoustic shadow’ within the kidney), IVU, or CT urogram (CTU).
Ureteric calculi: sensitivity for detection of renal calculi is in the order of 50-70% (i.e. the false negative rate is between 30 and 50%; it misses ureteric stones when these are present in 30-50% of cases). CTU or IVU, which relate the position of the opacity to the anatomical location of the ureters, are required to make a definitive diagnosis of a ureteric stone. However, once the presence of a ureteric stone has been confirmed by another imaging study (CTU or IVU) and as long as it is radio-opaque enough and large enough to be seen, plain radiography is a good way of following the patient to establish whether the stone is progressing distally, down the ureter. It is not useful for ‘following’ ureteric stones that are radiolucent (e.g. uric acid), small (generally a stone must be 3-4mm to be visible on plain X-ray), or when the stones pass through the ureter as it lies over the sacrum. Ability of KUB X-ray to ‘see’ stones is also dependent on amount of overlying bowel gas.
Plain tomography (a plain X-ray taken of a fixed coronal plane through the kidneys) can be useful, but is rarely done nowadays with the availability of ultrasound and CT.
Opacities that may be confused with stones (renal, ureteric) on plain radiography: calcified lymph nodes, pelvic phleboliths (round, lucent centre, usually below the ischial spines).
Look for the psoas shadow—obscured where there is retroperitoneal fluid (pus or blood; Fig. 3.4).
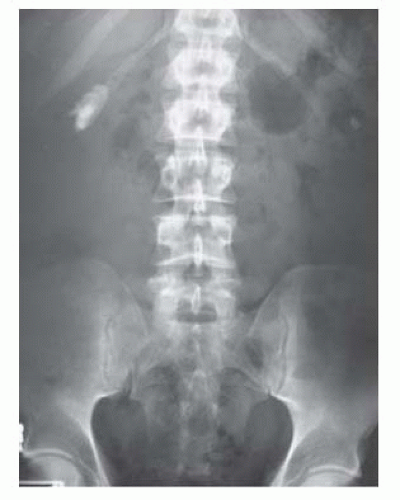 Fig. 3.3 Small staghorn calculus on KUB X-ray.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|