In Vitro Fertilization: Role of the Uterus
Michael D. Scheiber
The last two decades have witnessed both the birth and rapid evolution of the assisted reproductive technologies (ART). These technologies have revolutionized the care of the infertile couple. Yet, despite rapidly increasing pregnancy rates, the role of the uterine environment in the establishment of successful pregnancy remains ill defined. The clinical application of in vitro fertilization (IVF) as a common treatment for both female and male infertility has provided a unique opportunity for the study of the interaction between the developing embryo and the endometrium. This focus of reproductive research has resulted in the identification of numerous cellular and molecular biomarkers that help define uterine receptivity and may control the endocrine, paracrine, and autocrine communication processes during this critical period of implantation. Yet the precise coordination of events that allows the specific maturational development of the endometrium to provide a suitable environment for the dividing blastocyst while limiting the highly aggressive invasion of the placental trophoblasts in humans remains to be elucidated.
The wide variability in species-specific requirements for successful implantation makes it difficult to generalize the greater knowledge of the role of the uterus in implantation derived from studies in laboratory animals, largely rodents, to the human population. Yet, the advancement of research in humans will always be limited by the obvious practical, increasing legal, and ethical constraints that prevent the generation of controlled data regarding embryo implantation. This chapter strives to provide a brief overview of what is known regarding the role of the uterus in IVF.
The Endometrium and the Window of Implantation
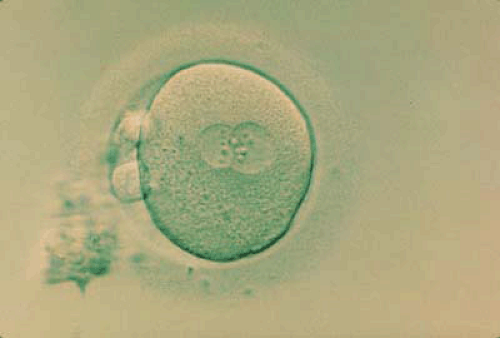
Fertilization of the human egg normally occurs in the fallopian tube within a limited time period following ovulation. The conceptus contains haploid male and female pronuclei, frequently referred to as the “2PN stage” by embryologists (Fig. 4.1), that fuse to form the diploid nucleus of the zygote. Nuclear division of the zygote without cytoplasmic growth results in the development of the multicellular morula on approximately day 4 after fertilization. Shortly after this, cavitation of the morula allows formation of the blastocyst (Fig. 4.2) that enters the uterine cavity on about the fifth day of development.
The window of implantation is that period (widely regarded as approximately days 6 to 10 postovulation) when the uterus is receptive to the blastocyst and its nidation. Apposition of the blastocyst follows its entry into the uterine cavity. And although this stage is poorly understood, pinopodes (microprotrusions of the apical surface of the luminal epithelium) extract uterine fluid through the process of pinocytosis and allow contact between the blastocyst and the underlying epithelium. Apposition is followed by embryonic attachment and then epithelial penetration and invasion by the trophoblasts.
Progress with IVF has allowed for a more precise evaluation of the human window of implantation. By studying successful implantation in women undergoing frozen embryo transfers and in those receiving embryos generated from donated oocytes, the synchronicity of the embryonic stage of development and the receptivity of the uterus has been elucidated. Numerous protocols exist in clinical practice to create an “artificial endometrium” via exogenous hormone replacement in preparation for transferring frozen embryos or embryos generated from donated oocytes. Oral, transdermal, intramuscular, and intravaginal estrogen have all been used with good success to stimulate endometrial development. Progesterone supplementation is then provided by either the intramuscular, vaginal, or oral route.
The various studies summarizing embryonic-uterine synchronicity have been nicely summarized by Rogers and Leeton. The available data suggest that the implantation window in humans is probably at least 3.5 days, but may extend to 7 days. The optimal time to transfer cleaved human embryos in IVF cycles dependent on exogenous hormones is probably between 3 and 5 days following the initiation of progesterone therapy. Recent advances in embryo culture media have allowed the emergence of blastocyst embryo transfers in many IVF centers, and progesterone therapy must be extended by 2 to 4 days to allow for the more
advanced stage of embryo development when using this technique. Because there is no contribution from the corpus luteum in these specific assisted reproductive techniques, estrogen and progesterone supplementation must be continued until past the time of the traditional luteal-placental shift at 8 to 9 weeks of gestation. Exogenous progesterone supplementation is typically continued until 9 to 12 weeks of gestation to allow for an adequate margin of safety.
advanced stage of embryo development when using this technique. Because there is no contribution from the corpus luteum in these specific assisted reproductive techniques, estrogen and progesterone supplementation must be continued until past the time of the traditional luteal-placental shift at 8 to 9 weeks of gestation. Exogenous progesterone supplementation is typically continued until 9 to 12 weeks of gestation to allow for an adequate margin of safety.
Endometrial Biomarkers of the Window of Implantation
The seminal work of Noyes et al. demonstrated the daily morphologic changes of the endometrium associated with the menstrual cycle several decades ago. Since that time, molecular studies have led to a rapid expansion in the numbers of gene products that have been identified to be cycle specific and perhaps critical to the process of embryonic implantation. This area of research has been well summarized by a number of authors.
As mentioned earlier, pinopodes appear during a variable 4- or 5-day period during the secretory phase of the menstrual cycle for 1 or 2 days and play a role in the interface between the epithelium and the blastocyst. Pinopode density may correlate with implantation.
Cell adhesion molecules (CAMs) including the integrins, cadherins, selectins, and the immunoglobulin superfamily have been implicated in all stages of reproduction from fertilization to implantation and placental development. The best studied of these are the integrins, of which at least 20 heterodimers have been identified. An effort to replace the criteria of Noyes et al. for endometrial dating with integrin expression was not found to be clinically useful. Nevertheless, patients with unexplained infertility, hydrosalpinges, endometriosis, and polycystic ovary disease have all demonstrated aberrant integrin expression that may be associated with poor IVF implantation rates. Glycodelin has also been shown to peak during the putative implantation window in exogenous hormone replacement cycles for donor oocyte IVF.
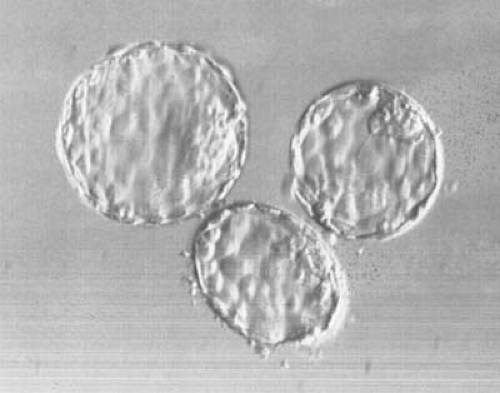 FIGURE 4.2 Human blastocysts on day 5 after in vitro fertilization. The inner cell mass as well as the trophectoderm are seen. (Courtesy of Erica J. Behnke, PhD, HCLD.) |
Several different cytokines may play a role in modulating endometrial receptivity. It has been postulated that the cytokine colony-stimulating factor (CSF)-1 plays a role in facilitating blastocyst attachment since it is abundantly expressed in the endometrium and mice with a knockout for the CSF-1 gene have reproductive alterations and are infertile. Interleukin-1 is involved in embryo attachment and implantation at least in the mouse model, and altered expression of leukemia inhibitory factor (LIF) has been associated with infertility as have mutations in the LIF gene coding region in infertile women. The glycoprotein MUC1 may also play a role in mediating implantation in many species, and endometrial MUC1 deficiency may be associated with recurrent pregnancy loss.
Jones et al. identified nine different chemokines in human endometrium that have varied up-regulation of messenger RNA (mRNA) during endometrial receptivity and early pregnancy. Meanwhile, the endometrium has been identified as a target tissue for leptin action, whereas leptin mRNA is expressed at the blastocyst stage, indicating a potential role in the endometrial-blastocyst interaction.
Although the identification of these biomarkers of uterine receptivity has helped to define the complex cellular interactions that regulate implantation, the clinical utility of this knowledge has unfortunately not yet come to fruition. Little can be done yet at the bedside to replace or control disordered regulatory mechanisms. Continued work in the clinical application of this basic science knowledge has tremendous potential.
The Effects of Ovarian Stimulation on Endometrial Receptivity
Although IVF pregnancy rates have improved dramatically over the last decade, implantation rates still remain relatively poor (approximately 15% to 25%) despite the transfer of apparently healthy embryos. Although other underlying factors, such as egg and embryo quality, age, and transfer techniques, can all clearly make a difference, a significant portion of implantation failures are probably due to altered endometrial receptivity, perhaps as the direct result of ovarian stimulation or the indirect result of the subsequent abnormal steroid hormone environment.
The combination of clomiphene citrate (CC) and human menopausal gonadotropins (hMG) was once a popular choice for ovarian stimulation in preparation for oocyte retrieval and IVF-embryo transfer. Endometrial biopsies done in women receiving this stimulation protocol often display an advanced pattern (endometrial dating greater than expected) that may result in an abnormal endometrial-blastocyst interaction. In an IVF cycle, the day of oocyte retrieval is usually artificially designated day 14. CC has also been postulated to create a luteal phase defect and have antiestrogenic effects on the uterine lining. Recently CC has also been shown to cause aberrant endometrial integrin expression as well as a failure of the normal progesterone receptor down-regulation during the window of implantation in normo-ovulatory women. A recent trend toward replacing CC with the aromatase inhibitors may help to avoid some of the pitfalls associated with CC use, as anastrazole plus FSH stimulation can have a positive effect on murine implantation.
Most recent studies of the endometrial morphology in stimulated IVF cycles include the use of gonadotropin-releasing hormone agonists (GnRHa) in combination with hMG or FSH and have been well summarized by Bourgain and Devroey. Implantation rates in cycles using GnRHa are generally superior to those from stimulations without GnRHa. Endometrial biopsies in the preovulatory phase before any rise in serum progesterone of such stimulated cycles showed accentuated proliferative aspects and early secretory changes, whereas periovulatory retrieval-day biopsies generally showed endometrial advancement of 2 to 4 days. In contrast to these studies, in a program with unusually high pregnancy rates among its infertile younger patients, no difference was noted between implantation, pregnancy, and delivery rates between IVF with embryo transfer (IVF-ET) patients and recipients of ovum donation, suggesting that the effects of ovarian stimulation on the developing endometrium may have minimal clinical significance.
The advent of clinically acceptable GnRH antagonists (GnRHA) has led to their use in IVF programs around the world. Endometrial biopsies performed on the day of oocyte retrieval in FSH-GnRHA cycles demonstrated advanced dating in all patients. No pregnancies were established in a series of 55 patients if the uterus was more than 3 days out of phase on the day of ovum pickup.
Direct studies on the endometrium in the luteal phase following IVF-ET are limited for obvious reasons, but most programs do support the luteal phase with exogenous progesterone (intramuscular [IM], vaginal, or oral). However, no randomized control trial has been conducted to evaluate the necessity of this approach. Instead of progesterone supplementation, many programs will use a second luteal-phase “booster shot” of human chorionic gonadotropin (hCG) 5 to 7 days after oocyte retrieval to stimulate progesterone production from the corpora lutea, but this approach may place the stimulated patient at greater risk for ovarian hyperstimulation.
The altered endometrial environment in stimulated cycles may result from high levels of serum estradiol exposure, an early and increased exposure to progesterone, or possibly even the direct effects of hCG or GnRH agonists or antagonists on the endometrium. Whatever the causes, an altered endometrial environment may partially account for the persistent relatively poor implantation rates observed with current assisted reproductive techniques.
Clinical Evaluation of the Endometrium During Assisted Reproduction
The importance of the endometrial environment in implantation rates has led to the pursuit of clinically useful modalities by which to predict a uterine environment favorable to embryo implantation in the cycling IVF patient. Most of these imaging modalities use ultrasound.
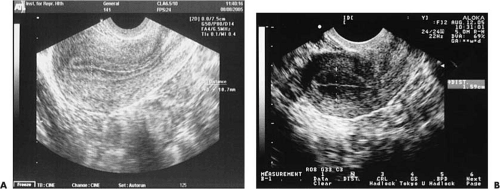
The endometrium displays a hormonally responsive thickening on ultrasound throughout the cycle. The maximum endometrial thickness can be measured in the longitudinal view as the perpendicular distance between the two echogenic interfaces of the endometrial-myometrial junctions near the fundus (Fig. 4.3) and may be correlated with endometrial receptivity. Overall thickness of the endometrium can help predict pregnancy rates, with a minimal thickness of 5 to 7 mm seeming to be necessary for implantation to occur successfully, with rare exceptions. It should be noted that the utility of this measurement in predicting pregnancy outcome from IVF-ET is not without its opponents.
Perhaps more important than actual thickness is the ultrasonographic appearance of the uterine lining. The triple-stripe or trilaminar pattern of echogenicity (Fig. 4.4) is associated with the highest pregnancy outcomes in IVF-ET cycles. Review of 117 oocyte donation cycles showed that 91% of conception cycles demonstrated a triple-stripe pattern on ultrasound compared with 44% of nonconception cycles.
Uterine artery blood flow measurements as a predictor of uterine receptivity in IVF have been largely disappointing. The pulsatility index (PI) on or near the day of embryo transfer generally cannot predict the likelihood of pregnancy in assisted reproduction, although a PI value of >3 to 3.5
may indicate a poor chance of conception. Uterine artery PI is problematic because it is more representative of total uterine environment (which is largely myometrium) rather than the true endometrial environment.
may indicate a poor chance of conception. Uterine artery PI is problematic because it is more representative of total uterine environment (which is largely myometrium) rather than the true endometrial environment.
Uterine volume has been examined as a predictor of uterine receptivity in IVF-ET with mixed success. Decreased implantation rates were noted with sonographic uterine volumes of <2 to 2.5 mL for IVF-ET or ovarian stimulation with intrauterine insemination. A minimum volume of 1.59 mL for pregnancy was noted in 135 patients undergoing IVF-ET, but endometrial volume overall did not allow for successful prediction of subsequent IVF outcome.
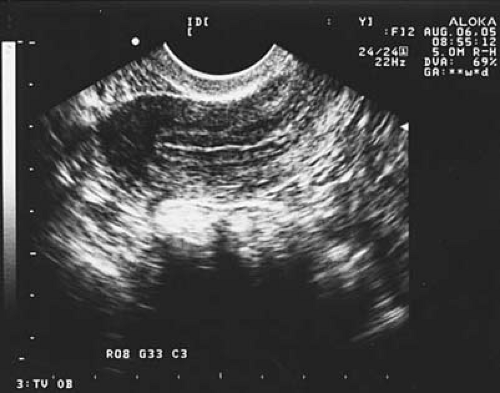 FIGURE 4.4 A trilaminar or triple-stripe appearance to the uterine lining on two-dimensional transvaginal ultrasound is associated with favorable implantation following IVF-ET. |
Several authors have examined the utility of magnetic resonance imaging (MRI) in describing the uterine anatomy in the normal menstrual cycle, but limited information exists on the MRI appearance of the endometrium during ovarian stimulation. The above discussion clearly indicates that an ideal imaging modality with high predictive values for IVF-ET pregnancy outcome has yet to be identified.
Clinical Uterine Factors Affecting IVF-ET
Although the basic science behind successful uterine preparation and endometrial receptivity is a fascinating area, the average practitioner is still left with the patient undergoing IVF-ET and the challenge of safely optimizing pregnancy rates with every procedure. Therefore, it is important to consider clinical uterine factors that may affect uterine receptivity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree