Fig. 4.1
CT scan, native axial, and volume rendering (VR) reconstruction. On axial plane, the lacrimal sac is a rounded structure enclosed between the anterior and posterior lacrimal crest (arrows). VR better delineates the fossa created by these crests (arrowhead).
MRI fails to depict the fine details of bone anatomy, even when 3D sequences – with high matrix and submillimetric pixel size – are used; this is partly due to the lower spatial resolution achievable (as compared with radiologic techniques) and partly due to the very low signal intensity displayed by cortical bone. The lacrimal sac is displayed as a T2 hyperintense, T1 hypointense, and enhancing structure; central hypointensity may be seen on all pulse sequences when the sac is filled with air (Fig. 4.2).
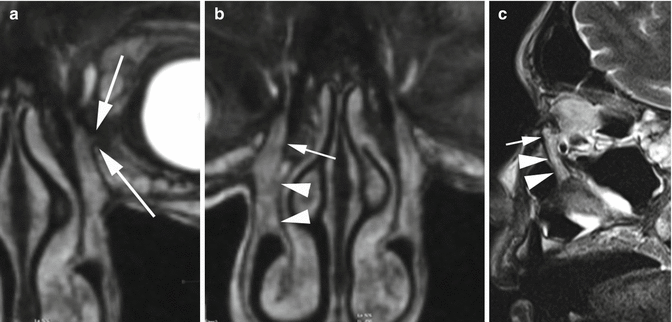

Fig. 4.2
MRI anatomy of the lacrimal pathway. 3D GE T1 with contrast on coronal plane (a, b) and sagittal TSE T2 (c). Although as a general rule the canalicula are below the detection of cross-sectional imaging, this coronal oblique reconstruction shows the junction between canalicula and lacrimal sac (arrows in a). The lacrimal sac (small arrows in b and c) and nasolacrimal duct (arrowheads in b and c) are – on opposite – fully delineated
The lacrimal sac directly continues in the NLD, the terminal tract of the lacrimal pathway. NLD mainly runs into the nasolacrimal canal (NLC), a vertical bony canal bordered anterolaterally by the maxillary bone (lacrimal sulcus of frontal process and medial maxillary sinus wall) and posteromedially by lacrimal bone and inferior turbinate. NLD is easily seen with both CT and MRI displaying density/signal intensities similar to the lacrimal sac. However, again axial CT (CBCT) scans better display fine bony details of NLC, such as the close relationships between the posterior aspect of the NLC and the uncinate process.
Mean diameter of the NLC measures approximately 4 mm, generally with no significant difference between the two sides. On opposite, a statistically relevant difference in size (anteroposterior and transverse diameter, axial area) was shown between males and females; in addition, in females, the NLC courses at a more acute angle with nasal floor [11, 12].
The final tract of the NLD runs into the submucosa of the inferior meatus, to end at the valve of Hasner (Fig. 4.2). This final tract is, again, much better investigated with DCG and basically indistinguishable from the adjacent soft tissues on CT and MRI.
4.3 Imaging Findings in Nasolacrimal Duct Obstruction
4.3.1 Nonneoplastic Conditions
Dacryocystitis is the most common presentation of a nasolacrimal infectious process. CT and MRI findings of dacryocystitis are rather typical; the dilated lacrimal sac appears as a well-defined expansile lesion at the medial canthal region with low CT density (higher values are seen when pus formation occurs) (Fig. 4.3). Similarly, on MRI, T2 hyperintensity and T1 hypointensity indicate fluid content; slight T1 hyperintensity and restriction on DWI sequences herald purulent collection. After contrast administration, both techniques show peripheral enhancement of the lacrimal sac, reflecting inflammatory changes of the wall, and variable degrees of enhancement of preseptal soft tissues [13].
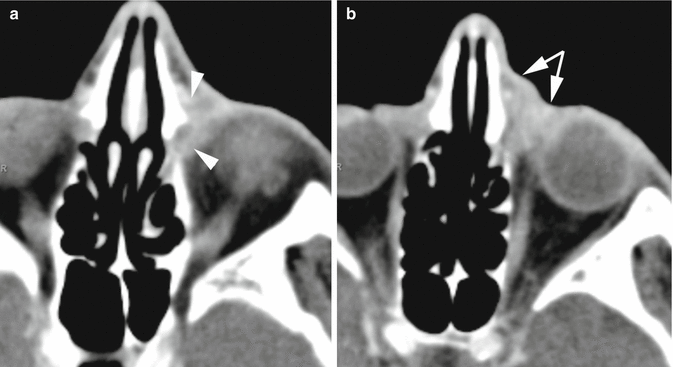

Fig. 4.3
Contrast-enhanced axial CT scan. Dacryocystitis. (a) The hypodense (fluid-filled) lacrimal sac is dilated and its walls are thicker than normal, enhancing and ill-defined from the surrounding fat tissue (arrowheads). (b) Thickening and enhancement of preseptal soft tissue is also noted (arrows)
Dacryocystitis is generally due to blockage of tear flow along the lacrimal pathway, resulting in tear stasis that favors bacterial proliferation. NLD obstruction is categorized into three classes, namely, congenital and acquired, of either primary or secondary origin.
Congenital NLD obstruction is most frequently secondary to the failure of canalization at the level of Hasner valve. This results in NLD dilatation, seen on cross-sectional scans as a cystic mass obstructing the inferior meatus [14, 15] with low CT density, T2 hyperintense signal on MRI, and lack of contrast enhancement (on both techniques) (Fig. 4.4).
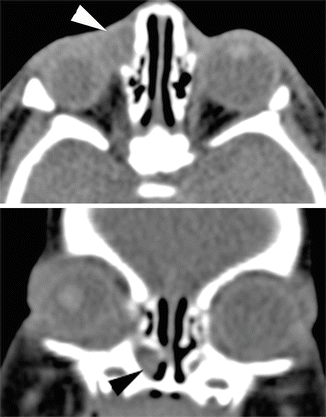

Fig. 4.4
Axial and coronal CT scan. The dilated NLD appears as a rounded cystic mass in the inferior meatus (black arrowhead). Dacryocystocele is also seen (white arrowhead)
Less commonly, retrograde distention of the lacrimal sac may produce a valve effect on Rosenmuller valve (junction between canalicula and lacrimal sac), resulting in dacryocystocele formation [16]. In this case, both CT and MRI demonstrate, along with a cystic mass in the inferior meatus, dilatation of the entire nasolacrimal pathway.
CT density and MRI signal intensity indicate fluid content; however, in the case of superinfection, CT density of the fluid increases whereas spontaneous T1 hyperintensity and restriction on DWI sequences may be seen [17].
From an imaging standpoint, primary acquired NLD obstruction is a diagnosis of exclusion. Few of the many predisposing factors that have been evoked in the literature to explain this condition (most of which are indeed quite controversial) [18] have an imaging counterpart. Chronic inflammation of the paranasal mucosa is nicely shown by cross-sectional studies. However, patients should never be scanned before completion of a cycle of medical treatment, to better discriminate chronic inflammation from bouts of reacutization.
Gender-related differences in NLD anatomy (see above) have been studied mainly with CT or MRI; however, the information provided on this aspect are more relevant on a wide scale than in the assessment of the individual patient.
The main roles of imaging in primary NLD obstruction are thus the demonstration of the site and extension of the stenosis and the exclusion of a secondary cause (Fig. 4.5).
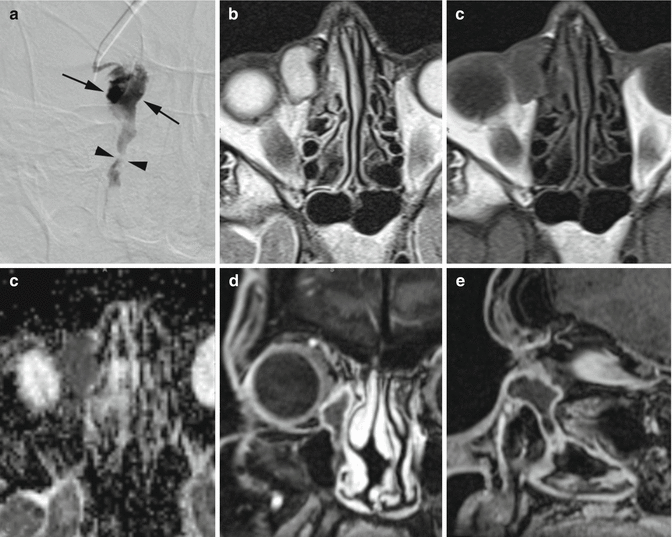

Fig. 4.5
DCG (a) shows partial obstruction of the right NLD (arrowheads) and lacrimal sac dilatation (arrows). On MRI, the lacrimal sac shows T2 hyperintensity (b) and T1 hypointensity (c), compatible with fluid content. The restriction on DWI sequence (d) however indicates the presence of pus or cellular debris. Coronal (e) and sagittal (f) reformation of 3D GE T1 with contrast rule out the presence of a mass lesion obstructing the lacrimal pathway
DCG plays a primary role, enhanced by the possibility to combine the diagnostic time with a therapeutic interventional procedure (see below), ultimately offering one-stop-shop management.
Secondary NLD obstruction may be due to noninfectious inflammatory diseases, chemotherapy, cocaine abuse, foreign bodies (dacryoliths), or trauma [19] (Fig. 4.6).
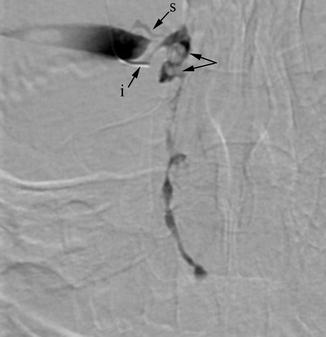

Fig. 4.6
DCG. Dacryoliths are seen as small filling defects (arrows) within a mildly dilated lacrimal sac. The NLD has irregular caliber but is patent. i inferior canaliculus, s superior canaliculus (retrogradely opacified)
Wegener’s disease and sarcoidosis may affect nasal and NLD mucosa producing a quite characteristic MRI pattern with T2 hypointense thickening of the submucosa. CT findings are much less specific, thus difficult to differentiate from chronic rhinosinusitis. Destruction of nasal septum and bone structures of the lateral nasal wall can be expected in Wegener’s disease and (much more frequently) in cocaine abuse lesions (Fig. 4.7).
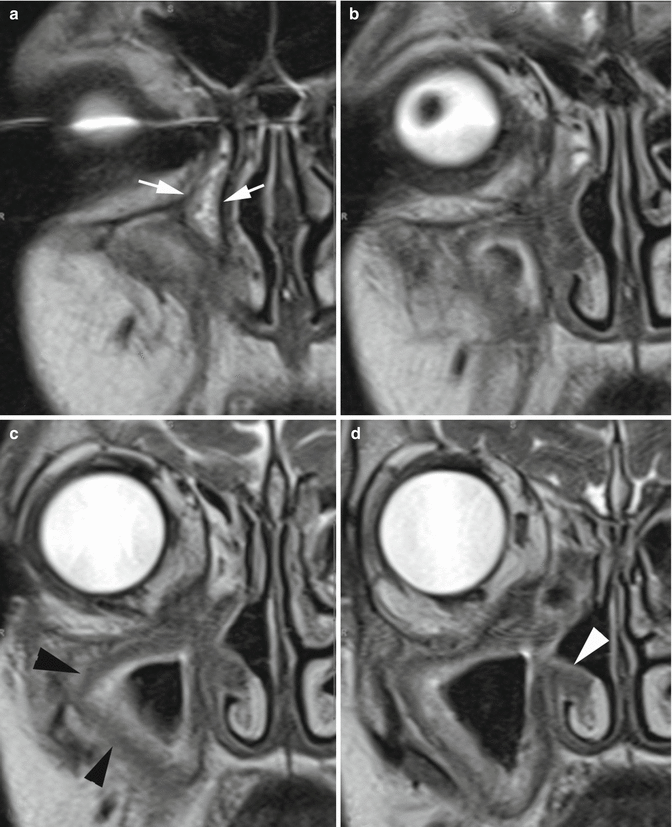

Fig. 4.7
MRI, coronal TSE T2. (a) Diffuse thickening of the mucosa (hyperintense signal) and submucosa (hypointense signal) of maxillary sinus (arrowhead in c) and NLD (arrows in a). Submucosal thickening of the inferior turbinate (arrowhead in d) and nasal fossae floor (b–d). Such pattern may suggest aggressive inflammatory disease such as Wegener’s granulomatosis (confirmed in this case) or sarcoidosis
Posttraumatic obstruction may be due to either mucosal synechiae or bone fragments impinging on the lacrimal pathway.
Prompt surgical correction of fractures and NLD protection with tube stenting have decreased the frequency of posttraumatic obstructions. Strictures produced by bony wall changes or synechiae of the mucosal lining are generally best seen on DCG.
4.3.2 Neoplasms of the Lacrimal Pathway
Neoplasms of the lacrimal pathway are exceedingly rare; lacrimal sac tumors account for less than 1 % of tumors of the orbit (including adnexa) [20, 21]. As a consequence, few large series are available in the literature.
In a series of 115 lesions, Stefanyszyn et al. [22] found an almost even malignant/benign distribution (55 % vs 45 %) with prevalence of epithelial over non-epithelial hystotypes.
Cross-sectional imaging plays a limited role in the characterization of lesions. Most of the time, benign lesions appear as smooth, well-defined filling defects of the lacrimal sac. Large lesions may completely fill and expand the sac – though maintaining sharp borders – and remodel adjacent bone structures. On opposite, ill-defined margins with infiltrative pattern and bone destruction herald a malignant tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








