Fig. 2.1
(a) (Anatomie des parties de la generation de l’homme et de la femme Gauthier D’agoty Paris 1773 National library of Medicine, Jacques Fabien. Exposition anatomique des organes des sens, jointe à la névrologie entière du corps humain, et conjectures sur l’électricité animale et le siège de l’âme, par M. Dagoty père…. Paris : Demonville, 1775). (b) The nasolacrimal duct
This composite anatomo-functional unit has a complex sensory, motor, and excitosecretory neurological control. In particular, a great importance is the function of the trigeminal nerve, as regards the sensory function, and facial nerve, for the excitosecretory and motor function. In order for this complex system to be effective, the lacrimal drainage system performs a fundamental rule. Its failure produces not only epiphora but also compromises the functional balance of the entire system
This chapter summarizes recent advances in knowledge of the lacrimal system, subdivided in three parts:
1.
The lacrimal film and the ocular surface
2.
Eye-associated lymphoid tissue (EALT)
3.
Physiology of the lacrimal drainage system
2.2 Tear Film and Ocular Surface
The surface of the eye is an extraordinary and vital component of vision. The smooth, wet surface of the cornea is the major refractive surface of the visual system, which, along with corneal transparency, enables light to proceed through the lenses and into the retina, for photoreceptor activation. Unlike all other wet-surfaced epithelia of the body, the ocular surface is directly exposed to the outside world where it is especially subject to desiccation, injury, and pathogens. Maintenance and protection of the smooth refractive surface of the cornea is the function of the ocular surface system (OSS)[1] (Fig. 2.2).
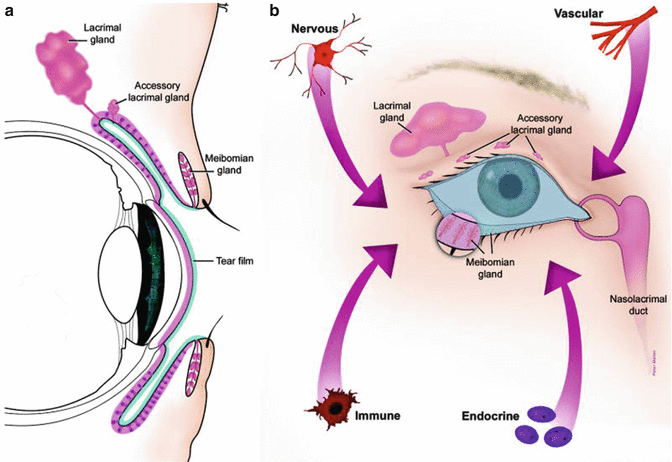

Fig. 2.2
(a) The ocular surface system. Sagittal section showing that the ocular surface epithelium is continuous (pink). (b) Frontal view of the ocular surface system, which includes the surface and glandular epithelia of the cornea, conjunctiva, lacrimal gland, accessory lacrimal glands, and meibomian gland
OSS is defined as the ocular surface, which includes the surface and epithelia of the cornea, conjunctiva, lacrimal gland, accessory lacrimal glands, and meibomian gland and their apical (tears) and basal (connective tissue) matrices; the eyelashes, with their associated glands of Moll and Zeis; and the nasolacrimal duct. All components of the system are linked functionally by continuity of the epithelia, by innervation, and by the endocrine, vascular, and immune systems. The rationale for the use of the term “ocular surface system” is severalfold. First, the primary, synergistic function of the system components is to provide, protect, and maintain a smooth refractive surface on the cornea. Thus, the term ocular surface system is linked to its primary function at the ocular surface. Second, all the epithelia at the ocular surface are continuous, with no breaks between regions, and all are derived from the surface ectoderm. The corneal and conjunctival epithelia are continuous, through the ductal epithelium, to the lacrimal glandular epithelium, as is the case with the accessory lacrimal glands, the meibomian gland, and the nasolacrimal system. Communication along these epithelia occurs through gap junctions and cytokines [2–4]. Third, all regions of the ocular surface epithelia produce components of the refractive tear film: the corneal and conjunctival epithelia produce hydrophilic mucins that hold tears onto the surface of the eye; the lacrimal and accessory lacrimal glands secrete water and a host of protective proteins; the meibomian gland provides the superficial tear lipid layer that prevents tear evaporation. The nasolacrimal epithelial system adsorbs tear components and is believed to, through its cavernous vascular system, control and regulate tear outflow, helping to maintain the appropriate tear level, as a result of a fine balance between secretion and outflow [5]. The functions of the various regions of the continuous epithelia and the eyelid blink are integrated by the nervous, endocrine, circulatory, and immune systems and are supported by the connective tissue with its resident cells and blood vessels. The smooth refractive tear film is maintained on the cornea and ocular surface by the blinking of the eyelid, to replenish the tears over the cornea, through continuous constitutive secretion of tear components by all areas of the ocular surface epithelia and by specializations on the apical surfaces of the corneal and conjunctival epithelia. Early hypotheses of tear film structure separated the secretions from the lacrimal gland, meibomian gland, and conjunctival goblet cell mucins into three separate, distinct layers within the tear film: the lipid, aqueous, and mucin layers, respectively (Wolff’s concept) [6]. While it is clear that the meibomian gland-derived lipid layer is partitioned on the surface of the aqueous layer, more recent data suggest that the aqueous tears are a mixture of lacrimal fluid and mucins, without a distinct mucin layer within the tears [7, 8] (Fig. 2.3).
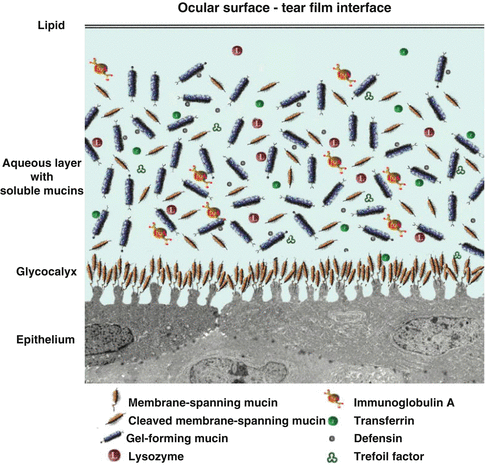

Fig. 2.3
Tear film and its interface with the ocular surface epithelium
The tear–epithelial cell interface is critical for tear film maintenance on the corneal and conjunctival epithelia. As with all wet-surfaced epithelia of the body, including those of the ocular surface system and the gastrointestinal, respiratory, and reproductive systems, maintenance of fluids on the cell surface is facilitated through membrane specialization on the apical surface membrane, where it abuts the luminal surface. A hydrophilic, heavily glycosylated glycocalyx is present on these apical surface membranes of the epithelia. At the ocular surface, the apical cell membrane adjacent to the tear film interface is thrown into short membrane folds, termed “microplicae” (Fig. 2.2). Membrane-associated mucins emanate from the tips of the “microplicae” and extend up to 500 nm from the membrane to form the glycocalyx. Membrane-bound mucins (MUCs 1, 4, and 16) of corneal and conjunctival epithelial cells are present in the glycocalyx layer. Soluble mucins (MUC5AC) from conjunctival goblet cells, as well as MUC5B and MUC7 from lacrimal glands, are in solution in the tear film [8, 9]. Both MUC5B and MUC7 have been shown to bind bacteria and contribute to innate immunity of the tear film. Besides MUC5AC, conjunctival goblet cells secret the trefoil family factor peptides (TFF peptides) TFF1 and TFF3 [10]. The TFF peptides are, together with mucins, typical constituents of mucus gels that influence the rheological properties of the tear film, promote migration of corneal epithelial cells, have antiapoptotic properties, and induce cell scattering [11]. Conjunctival and corneal epithelial cells are able to react against pathogens by the production of inducible antimicrobial peptides (a kind of body’s own antibiotics). In addition, in certain disease states, the corneal cells are able to produce TFF3.
The Table 2.1 shows the distribution of mucins and TFF peptides in the healthy lacrimal system.
Table 2.1
Anatomie des parties de la generation de l’homme et de la femme Gauthier D’agoty Paris 1773 National library of Medicine
Localization | Mucins | TFF peptides |
|---|---|---|
Lacrimal gland | MUC1 (membrane bound on acinar cells) MUC4, MUC5B, MUC7 (in acinar cells) MUC5AC (in excretory duct cells) | |
Cornea | MUC1, MUC4, MUC16 (membrane bound on epithelial cells) | |
Conjunctiva | MUC1, MUC4, MUC16 (membrane bound on epithelial cells) MUC5AC (in goblet cells) | TFF1, TFF3 (in goblet cells) |
Nasolacrimal ducts | MUC1, MUC4 (membrane bound on columnar cells) MUC2, MUC5AC, MUC5B (in goblet cells) MUC5AC, MUC5B (in intraepithelial glands) MUC7 (in columnar cells) | TFF1 (goblet cell associated) TFF3 (in columnar cells) |
2.3 Eye-Associated Lymphoid Tissue (EALT)
Mucosal organs represent a special moist compartment of the body’s surface that is equipped with a diverse array of defense mechanisms, in order to avoid microbial colonization, as is also true for the ocular surface. Besides the innate defense mechanisms, lymphoid cells are becoming increasingly recognized as playing an important part in the preservation of mucosal integrity. Lymphoid cells form a “mucosa-associated lymphoid tissue” (MALT) in these organs. MALT represents an accepted component in organs such as the intestine, respiratory system, or genital tract.
However, its presence at the normal human ocular surface is not fully recognized as yet; for example, the supply of the ocular surface with protective immunoglobulins is usually attributed to the lacrimal gland. Until relatively recently, immunohistological evidence for the presence of IgA-positive plasma cells and its transepithelial transporter molecule was controversial at the ocular surface, although the presence of plasma cells was always confirmed by histological studies. In the conjunctiva, for example, lymphoid cells occur in most parts, but they are interspersed as a thin, discontinuous, and inconspicuous layer into the epithelium and connective tissue of the lamina propria. This may be one reason why these cells were often overlooked. They have either not received much attention or they were assumed to be inflammatory infiltrations and not recognized as part of the mucosal immune system until the advent of mucosal immunology in other parts of the body. Secretory IgA (SIgA) forms a first line of defense at mucosal surfaces which also include the ocular mucosa, consisting of the ocular surface proper (conjunctiva and cornea) and its continuously connected mucosal adnexa composed of the lacrimal gland (LG) and lacrimal drainage system that together form an anatomical and functional unit. The ocular surface and lacrimal drainage system represent a mucosa, similar to that of the intestine and airways, along with a large associated gland, the lacrimal gland (LG). The ocular mucosa is directly and constantly exposed to the external environment, which puts it at risk of microbial invasion and allergic disease. To counter these environmental insults, the mucosa is supported by an array of defense mechanisms [12].
2.3.1 The Lacrimal Gland
The lacrimal gland (LG) has been an accepted part of the immune system since IgA was detected as the predominant immunoglobulin in human tear fluid. IgA and its transporter, termed secretory component (SC), were identified in the normal human lacrimal gland by immuno-morphological techniques [13]. Histologic sections of the human lacrimal gland confirmed the presence of numerous plasma cells located around the secretory acini. They formed a diffuse lymphoid tissue together with other leukocytes, mainly lymphocytes. In IHC (immunohistochemistry), the cytoplasm of the plasma cells stained intensely positive for IgA, whereas the acinar epithelial cells stained weakly, with increasing intensity toward the apical pole and at the luminal surface. SC was absent from the plasma cells but showed a more homogeneous and much stronger staining in the acinar cells than did IgA.
2.3.2 Excretory Lacrimal Ducts
The excretory lacrimal ducts that leave the gland and open into the conjunctiva have a cellular sheet of diffuse lymphoid tissue with the same characteristics as in the LGs. The cells in the sheet consisted mainly of plasma cells and lymphocytes. The immunostaining characteristics for IgA and SC were also present at the duct. IgA intensely stained in the periductal plasma cells and only weakly inside the ductal epithelium, mainly in the apical cytoplasm and at the luminal surface. The staining for SC was intense in the epithelium and mostly restricted to the superficial layer of the two- to three-layered pseudostratified epithelium.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








