Fig. 7.1
Epiphora in infant
If tearing is intermittent, and none of the above signs are present at the time of examination, the fluorescein dye disappearance (Fig. 7.2) test can be performed to help confirm the diagnosis [2]; this test is performed by placing fluorescein in the eye and observing the disappearance of the dye over time.

Fig. 7.2
Fluorescein dye disappearance test
7.2 Anatomic Site and Embryology
The development of the lacrimal outflow system begins with a thickened ridge of cells of surface ectoderm at the naso-optic fissure [1]. In the 12-week embryo, these cells dive into the surrounding mesoderm to form a solid cord of cells, elongating in a direction from the future medial canthus to the primitive nasal cavity. The canalization of the solid rod to form a hollow tube proceeds in a direction from the medial canthus to the nose and should be complete during the sixth month of gestation. The canaliculi open into the eyelid margin during the seventh month, just before the eyelids separate. The canalization of the ectodermal rods should be complete during the sixth month, but a thin membrane sometimes remains at the junctions of the tubes and may be responsible of the successive nasolacrimal duct obstruction. Postnatal sucking and respiration probably play an important role in the rupture of many persistent membranes. A study found that more than 70 % of stillborn infants have CNLDO at birth [3], many times higher than that seen in normal newborns.
Really other anomalies can take birth from different problems in every stage of the embryogenetic and development processes.
Agenesis of some parts of the drainage system may result from failure of parts of the surface ectoderm to invaginate. Partial canalization may result in a loss of patency or stenosis at any point in the system, including the puncta, lacrimal sac, and lacrimal duct.
7.3 Etiology
7.3.1 Congenital
Congenital nasolacrimal duct obstruction usually results in persistent tearing and is often responsible of infections, such as dacryocystitis, orbital cellulitis, and viral or bacterial conjunctivitis. The underlying cause of dacryocystitis remains controversial [1].
One argument states that obstruction is the primary cause, which leads to the accumulation of tears and cellular debris with secondary infection. An alternative argument states that infection is the primary event and is responsible of the secondary obstruction resulting in fibrosis and inflammation.
The most common organisms that are identified from children who have CNLDO are Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella spp., Streptococcus spp., Pasteurella spp., and Acinetobacter lwoffii.
Obstruction [4] can occur at any level along the lacrimal outflow pathway: at the punctum, canaliculus, lacrimal sac, nasolacrimal duct, or nasal ostium, but in infants [5] the most common cause of congenital nasolacrimal obstruction is a dysgenesis of the nasolacrimal duct during the embryonic period that is responsible of the presence of an imperforate membrane within the NLD at the level of the valve of Hasner or more proximal. The obstruction often resolves spontaneously, so the surgical treatment is limited to infants with epiphora and recurrent infections.
Although the natural history of congenital NLD is well documented, the mechanism underlying the delayed resolution of the obstruction in infants has not been elucidated.
An important cause of nasolacrimal duct obstruction in infants is the congenital dacryocystocele, which is characterized by the appearance of a cystic blue mass over the region of the lacrimal sac soon after birth [6]. It is thought to be the result of a persistent membrane at the level of the valve of Hasner and a functional obstruction of the common canaliculus or valve of Rosenmuller [1]. Dacryocystocele is unilateral in 86–100 % of cases [6]. The resulting lacrimal sac distention has been reported to be more common in female and non-Hispanic white patients, whereas familial cases have been described only rarely.
Congenital dacryocystocele is usually seen as an isolated abnormality [7]. Some authors [8] found an association between dacryocystocele and other congenital anomalies (Canavan disease and multicystic dysplastic kidney disease). Other authors [9, 10] reported the association with choanal atresia, cleft palate, and sphenoidal meningocele.
Lacrimal duct cysts [1] were described first by Raflo and colleagues in 1982 [11]. The cysts may obstruct the airways of infants, who are preferential nose breathers, which leads to respiratory distress that requires urgent surgical intervention.
Lacrimal fistulas, which may be congenital or acquired, occur in approximately 1 in 2000 live births [1]. Internal fistulas connect the lacrimal sac to the nasal mucosa and are diagnosed rarely. External fistulas connect the lacrimal system to the skin and are located most commonly inferomedial to the medial canthus. Occasionally lacrimal fistulas may be bilateral, have more than one opening, or end in a blind pouch. A fistula may become infected or cause local dermatitis from chronic drainage. Lacrimal fistulas have been reported in conjunction with several other abnormalities including nasolacrimal duct obstruction, mucocele, absent canaliculus, contralateral absent punctum, and total agenesis of the lacrimal system [1]. Several mass lesions may cause compression of the lacrimal outflow system and mimic nasolacrimal duct obstruction including meningoencephalocele, capillary hemangioma, dermoid cysts, sudoriferous cysts, nasal glioma, lymphangioma, lacrimal sac tumors, rhabdomyosarcoma, anterior ethmoiditis, and pneumatocele.
7.3.2 Acquired
The acquired type of NLD obstruction accounts for a relatively small number of cases of this condition in children [12].
The general categories of acquired obstruction causes are rare and include infections from bacteria (Staphylococcus aureus, Enterobacter, Treponema pallidum, Actinomyces, Propionibacterium, Fusobacterium, Bacteroides, Mycobacterium, and Chlamydia species), fungi (Aspergillus, Candida), and inflammatory, neoplastic, traumatic, and mechanical causes.
An important role is attributed to viral infections especially herpes simplex virus, varicella zoster virus, papilloma virus, and adenovirus. Some authors suggest that the obstruction is due to the damage of the substantia propria of the canalicular elastic tissue and/or the adherence of the inflammatory membranes to the raw epithelial surface of the canaliculus.
The authors of an interesting study reviewed the medical records of patients under the age of 15 years who had been treated with silicone intubation or dacryocystorhinostomy for NLD obstruction in a Korean hospital during 7 years (patients with a history of nasolacrimal duct trauma, those who failed previous probing for an isolated congenital NLD obstruction, and those with coexisting lacrimal drainage system anomalies or craniofacial defects were excluded) [12]. The leading cause of acquired NLD obstruction was EKC in 84.22 % of patients, idiopathic in 13.15 %, and ECK (epidemic keratoconjunctivitis) in 2.63 %.
7.4 Diagnosis
Radiologic imaging is very important to achieve a correct diagnosis of pediatric nasolacrimal duct obstruction [1].
Dacryocystography, the earliest radiologic method of evaluating the lacrimal system, is performed by injecting contrast material directly into the lacrimal system using a blunt cannula. Radiographs are taken with the patient supine and in Waters’ position as contrast is injected. DCG provides sufficient detail to localize the stenosis, diverticula, or fistulas within the lacrimal system and to visualize bony landmarks; however it provides poor detail of surrounding soft tissues.
Ultrasound is a simple and noninvasive method that can be used without sedation to reliably distinguish dacryocystoceles from other pathological conditions [13]. The sonographic appearance of a medial cystic mass, in communication with the dilated nasolacrimal duct, in addition to the typical fluid and debris content, pointed to the diagnosis of dacryocystocele.
Other more invasive imaging such as computed tomography or magnetic resonance imaging (MRI) are indicated if the US diagnosis is not conclusive (Fig. 7.3): CT has the advantage of detecting bone change; MRI has the advantage of characterizing the cyst contents without exposing the patients to radiation.
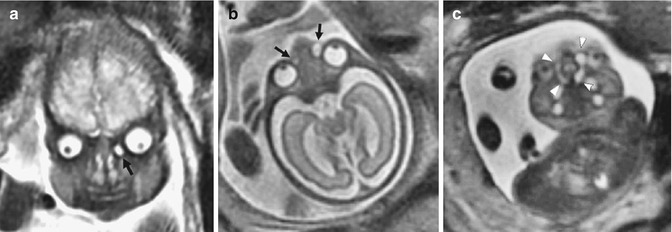

Fig. 7.3
MR images of dacryocystoceles. (a) Coronal SSFSE MR image of case 1 at 34 weeks’ gestational age shows unilateral dacryocystocele (black arrow). (b) Axial SSFSE image of case 6 at 27 weeks’ gestational age reveals bilateral dacryocystoceles (black arrows). (c) Paracoronal SSFSE MRI of the same fetus as in (b) shows dacryocystoceles continuing as enlarged nasolacrimal ducts and intranasal cysts (arrowheads) (Image taken from the article Yazici et al. [7])
It is possible to diagnose congenital dacryocystocele prenatally [7]. Prenatal US detection of dacryocystocele has been reported in a considerable number of cases [8, 14–16].
MRI is increasingly being used as an adjunct to prenatal US detection to evaluate fetal abnormalities, particularly those of the central nervous system and thorax; this modality, with its high contrast and spatial resolution, is superior to ultrasonography in imaging the fetal head and neck abnormalities.
7.5 Treatment Techniques
Nasolacrimal duct obstruction has a high rate of resolution without surgery. In an observational study by Paul [17], the rate of resolution of NLDO with medical management by 1 year of age was 80 % at 3 months, 70 % at 6 months, and 52 % at 9 months of age.
Medical treatment of NLDO consists of compression or massage of the nasolacrimal sac and topical antibiotics when a discharge is present. Medical management helps to ameliorate the symptoms while waiting for resolution and may increase the chance of resolution.
Massaging the nasolacrimal sac in a downward fashion will produce hydrostatic pressure which may rupture the membranous obstruction at the valve of Hasner.
In 1923, Crigler [18] described a maneuver to treat congenital NLDO. The Crigler maneuver was revisited recently by Stolovitch and Michaeli [19]. They found that the success rate of the Crigler maneuver was 56 % in children under 2 months of age, 46 % in children 2–6 months old, and 28 % in children older than 6 months. The authors recommended the Crigler maneuver for every infant presenting to a pediatric ophthalmologist with congenital NLDO at their initial office visit.
7.5.1 Probing
However after 12 months of age, the likelihood of spontaneous resolution decreases, and most patients are treated with probing of the nasolacrimal drainage system to open the blockage mechanically and with irrigation with dilute fluorescein solution to confirm patency (probing and irrigation). While typically performed under general anesthesia, intravenous propofol sedation may offer decreased risk, shortened operative times, decreased perioperative times, decreased perioperative morbidity, and lower operative costs [20].
7.5.1.1 Irrigation Test
Several recent studies have addressed the effectiveness of probing versus syringing (Fig. 7.4) and whether the treatments are effective in the older children [21–24].

Fig. 7.4
Irrigation test
Tahat [24] prospectively examined the success of irrigation, probing, or both in 300 eyes of 228 patients aged 12–13 months and found that probing or probing with irrigation was superior to irrigation alone (91 %, 96 %, and 64 % success rates, respectively). In a smaller study, Kim et al. [23] found probing to be statistically equivalent to irrigation with an antibiotic solution directed against cultured organisms, with success of around 90 %. Ciftci et al. [21] proposed a graded approach to treating NLDO in children, progressing from conservative therapy to irrigation, to probing, to silicone intubation. Using their protocol, they achieved a success rate of 100 % in children <2 years old and 94.5 % in children 2–6 years old.
Of cautionary note, Grech et al. [25] reported a case of bacterial endocarditis after lacrimal probing in a 3-year-old child with a history of ventricular septal defect, even though the child had received intravenous amoxicillin perioperatively. They recommend coverage with a first-generation cephalosporin, a fluoroquinolone or clindamycin, when infection is suspected at the time of probing. In high-risk patients, such as those with cardiac anomalies, coverage should consist of intravenous ampicillin with gentamicin.
Although nasolacrimal duct probing is a standard therapeutic procedure in the management of NLDO, some controversy exists regarding the optimal timing of probing and irrigation, outcomes in older children, and treatment of choice after a failed attempt. Some studies suggest that delaying the operation, especially after age 2, is associated with higher failure rates [26–28].
Many factors are believed to affect the success rate of nasolacrimal probing: age, bilaterality, prior failed probing attempts, prior failed conservative treatments, and dilated sacs have all been shown to significantly impact probing success (p < 0.05) [22].
Many studies have shown success rates to be higher at younger ages, especially among children <2 years of age [29–32]. The reason for the discrepant effect of age on probing success is unclear, even if the fibrosis that increases with age caused by the prolonged inflammation in lacrimal drainage system could represent the principal explanation [33, 34].
Another influencing factor in comparing the different probing success rates could be related to the type of procedure used. Repka et al. [28] reported that office-based procedures performed under topical anesthesia with physical restraints were associated with a significantly lower rate of success (72 %) when compared with procedures performed in a medical facility under brief general anesthesia (80 %). However other studies contradict these findings [35, 36].
Concerning outcomes of probing procedure, Arora et al. [37] reported an overall success rate of 72 %, while Honavar et al. [22] reported similar overall results with a success rate of 73 %, in a cohort of patients with 33 months of median age. The Pediatric Eye Disease Investigator Group (PEDIG)’s prospective study in 2008 showed a higher success rate (78 %) in a study population with a lower mean age (13.6 months) [28].
Maheshwari [38] reported a success rate of 81 % in children older than 2 years (mean age 45 months). Katowitz and Welsh [33] reported a probing success rate of 98.2 % in subjects aged 0–6 months, 95.9 % in subjects aged 7–12 months, 76.8 % in subjects aged 13–18 months, and 54.1 % in subjects aged 19–24 months. Similar results were reported by Mannor et al. [30].
7.5.2 Silicone Intubation
Nasolacrimal intubation involves probing the nasolacrimal duct, followed by placement of a silicone tube stent in one or both canaliculi. This procedure has been popular since its introduction in the late 1960s for the treatment of persistent NLDO after failed probing [39–48].
Intubation has also been used by clinicians for primary treatment of NLDO in older children or when the duct feels tight during probing [31, 41, 45, 49].
Nasolacrimal duct intubation consists of dilatation of at least one punctum and the placement of a monocanalicular or bicanalicular tube. Nasolacrimal intubation is performed in a surgical facility under general anesthesia and requires a single-use intubation set. The tubes may be removed in the office or in a facility with sedation [28].
Many intubation techniques and types of intubation sets have been described. One of the most commonly utilized stents is the bicanalicular Crawford stent in which steel probes with olive tips are used [50].
Monocanalicular intubation of the nasolacrimal drainage system has been introduced as another potential treatment option in NLDO [51]. The latter technique is less traumatic than bicanalicular intubation and relatively simple to perform. In addition, the tube does not require further manipulation after insertion except for the removal that may be easily performed in the office setting [52]. On the other hand bicanalicular tubes occasionally require sedation for surgical repositioning of a displaced tube and for removal.
Lacrimal tubes are usually removed after 2–6 months. Children under 2 years of age do well with tube removal at 6 weeks. Older children benefit from a longer period of intubation and may have an increased chance for success if the tube is left in place for at least 3 months [53].
Some recent studies analyzed the outcomes of nasolacrimal duct intubation.
Repka et al. [28], describing their results in a cohort of 139 patients treated with monocanalicular or bicanalicular intubation, reported a success rate of 91 %.
Lim et al. [49] reported a successful result in 104 of 122 (85 %) eyes treated with monocanalicular or bicanalicular intubation.
Engel et al. [54] analyzed the larger cohort (635 patients) treated by mean monocanalicular silastic intubation for NLDO. The authors obtained an overall success rate of 96.2 % with a recurrence rate of 3.8 % (with a median follow-up of 12 weeks).
Concerning time removal of the tube, Migliori and Putterman found that retention for only 6 weeks was sufficient for a satisfactory outcome [46].
Lim et al. [49] found that there was a significant decrease in success with retention of the tubes beyond 12 months. Conversely, other authors have found that retention for 6 months or more is preferable for an improved chance of success [52, 55].
7.5.3 Role of Endoscopy in Probing and Intubation
As lacrimal probing is a “blind” procedure, the addition of nasal endoscopy allows the examiner to identify successful passage of the probe thorough the lacrimal system and conversely the creation of false passages, as well as to identify and treat coexistent nasal pathology that may decrease the success of probing or intubation, such as close approximation of the inferior turbinate to the lateral nasal wall. Several recent studies have addressed the role of nasal endoscopy during lacrimal probing or intubation in children [58–61].
MacEwan et al. [60] identified a false passage rate of 15 % by nasal endoscopy, allowing redirection of the probe without the need for a second procedure. In children in whom the procedure could not be successfully passed, the procedure was converted to a DCR, allowing definitive treatment at the same setting. Therefore, nasal endoscopy at the time of probing may minimize the number of repeat procedures performed.
7.5.4 Endoscopic Dacryocystorhinostomy (DCR)
External [62–64] and endonasal DCRs [59, 65, 66] in children are highly effective procedures for the correction of a common NLDO unresponsive to medical therapy, probing(s), and intubation.
Nevertheless, the decision which one of these approaches should be used usually depends on the surgeon’s experience with EDCRs or EXT-DCRs and on the collaboration between ophthalmologists and otorhinolaryngologists familiar with the endonasal sinus surgery [66].
External dacryocystorhinostomy (DCR) for the treatment of nasolacrimal duct obstruction was first described by Toti in 1904 [67].
Caldwell described the first endonasal operative approach to the lacrimal system in 1893 [68].
However review of literature reveals paucity of data on the role of endoscopic DCR in children [72–76].
Pediatric DCR has some particularities inherent to the group of patients.
The anatomy is specific. The pediatric nasal airways are very narrow. The inferior turbinates are more bulky, and the nasal septum can be deviated making more difficult the access to the lacrimal eminence. A septoplasty is not recommended at this age because of possible impact on the facial growth. The agger nasi cell is not as well pneumatized as in adults. Identification of the lacrimal eminence and the uncinate process can be more difficult in such a narrow space. Transillumination of the lacrimal sac with a light probe can be of some help. The dissection is done at the level of the nasolacrimal duct, rather than the lacrimal sac.
Craniofacial abnormalities are more common in children than in adults [30, 77, 78]. Preoperative sinus CT scan is therefore recommended in the preoperative diagnostic workup.
Intraoperatively, the procedure can be bloodier than in adults mainly at the beginning of the surgery, when the nasal mucosa is resected. The suction is less effective in children because of the smaller diameter of the cannula. Decongestion of the nasal mucosa is less important than in adults. Adrenaline is used topically only. Infiltration with lidocaine plus adrenaline is contraindicated in a very young child. The dose must be calculated by the anesthesiologists according to the weight of the child to avoid an overdose.
The use of a cautery during the surgery can be of some help. Laser surgical devices can be used [79] but their cost is very high and it does not currently appear to improve results [80, 81].
A specific instrumentation is necessary. Micro “forceps, suction, curettes, and scissors” are necessary. Eloy et al. [82] recommend a 2.7 mm rigid telescope, while Cunningham [77] found out that a 4.0 mm rigid telescope is preferred in small children aged under 1 year because of its better illumination and wider field of vision. Lens-cleaning systems are too bulky to be used in the nose of very young children. The resection of the frontal process of the maxilla is usually started with a bone resector (Kerrison forceps).
The microdebrider with a dedicated diamond drill for DCR could only be used in the oldest children [83].
Concerning radiologic investigation, some authors recommend using CT prior to lacrimal surgery [69, 82].
Eloy et al. [82] affirm that CT scan is needed if a craniofacial abnormality is evident or if there is a history of skull fractures or previous sinus or skull base surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








