Jeffrey B. Kopp, Saraladevi Naicker
Human Immunodeficiency Virus Infection and the Kidney
Human immunodeficiency virus (HIV) infection is the defining infectious disease of our era. The 2012 UNAIDS report estimated that there are 35.3 million people living with HIV infection and 2.3 million new infections1; there have been 16 million deaths since the start of the epidemic. With regard to nephrologic issues, HIV-1 infection is associated with glomerular and tubulointerstitial disease, and patients with HIV infection are also at risk for nephrotoxicity from antiretroviral therapy (ART) as well as from other medications. Coinfections with other pathogens, in particular hepatitis B virus (HBV) and hepatitis C virus (HCV), can complicate the clinical picture. Acute kidney injury (AKI) and interstitial nephritis, resulting from medication and opportunistic infection, are not uncommon. As patients with HIV disease live longer with ART, patients are experiencing the complex, interacting effects of HIV infection itself, ART, and the worldwide diseases of development, including atherosclerosis, metabolic syndrome, type 2 diabetes, and end-stage renal disease (ESRD). Patients with HIV disease and chronic kidney disease (CKD) and ESRD, those undergoing chronic dialysis therapy, and those undergoing kidney transplantation face particular issues and concerns.
In addition to HIV-1, HIV-2 can cause immunodeficiency but rarely causes kidney disease. In this chapter, we use HIV to refer to HIV-1.
Human Immunodeficiency Virus–Associated Kidney Disease
The evaluation of AKI or CKD in a patient with HIV infection resembles that in other settings: history, focused particularly on medication use and other infections; physical examination; examination of the urine sediment; serum and urine chemistries; and, in many cases, renal imaging studies. Urine chemistries may include 24-hour timed or spot urine measurement of protein and albumin (for suspected glomerular disease), glucose, phosphate, and uric acid (for suspected proximal tubular disease). Indications for kidney biopsy include AKI without clear associated cause, especially with a nephritic sediment; nephrotic proteinuria; clinical evidence of thrombotic microangiopathy (TMA); and unexplained CKD. In the past, some clinicians argued that nephrotic proteinuria in an individual of African descent is likely to be HIV-associated collapsing glomerulopathy (CG) and that a biopsy is not necessary. The widespread use of ART in the current era makes this decision less tenable, and renal biopsy appears to be indicated to guide prognosis and therapy, particularly when substantial proteinuria is present.
Glomerular Disorders
Human Immunodeficiency Virus–Associated Collapsing Glomerulopathy
Human immunodeficiency virus infection is associated with various glomerular disorders (Table 58-1). The classic glomerulopathy of HIV infection is CG. The pathologic changes were described in early reports as focal segmental glomerulosclerosis (FSGS) and more recently as HIV-associated nephropathy (HIVAN), but the latter term has also been used to describe other glomerular pathologic processes associated with HIV infection.
Table 58-1
Kidney diseases associated with human immunodeficiency virus (HIV) infection.
Shown are diseases that are associated with HIV infection or its treatment. ART, Antiretroviral therapy.
| Kidney Diseases Associated with HIV Infection | |||
| Entity | Frequency | Associations | |
| Glomerular | Collapsing glomerulopathy | Common | African descent; particularly advanced HIV disease |
| Immune complex glomerulonephritis | Common | European, Asian descent, and black Africans in Africa | |
| Thrombotic microangiopathy | Uncommon | ||
| Membranoproliferative glomerulonephritis, with or without cryoglobulin-associated vasculitis | Rare* | Hepatitis C; enfuvirtide | |
| Membranous nephropathy | Rare* | Hepatitis B | |
| Fibrillary and immunotactoid glomerulopathies | Rare* | ||
| Amyloid nephropathy (AA type) | Rare* | ||
| Minimal change nephropathy | Rare* | Nonsteroidal anti-inflammatory medication | |
| Tubular | Acute kidney injury | Moderately common | Aminoglycosides, cidofovir foscarnet |
| Proximal tubule injury (Fanconi syndrome) | Moderately common | Tenofovir, adefovir, cidofovir, didanosine | |
| Diabetes insipidus | Uncommon | Amphotericin, tenofovir, didanosine, abacavir | |
| Chronic tubular injury | Moderately common | Amphotericin, cidofovir, adefovir, tenofovir | |
| Crystal nephropathy | Uncommon | Indinavir, atazanavir; sulfadiazine, ciprofloxacin, intravenous acyclovir | |
| Interstitial | Interstitial nephritis | Uncommon | Allergy to β-lactam, sulfa, ciprofloxacin, rifampin, proton pump inhibitor, allopurinol, phenytoin; also causes of crystal nephropathy listed above BK virus; generally advanced disease Immune reconstitution inflammatory syndrome; generally advanced disease; following initiation of ART |
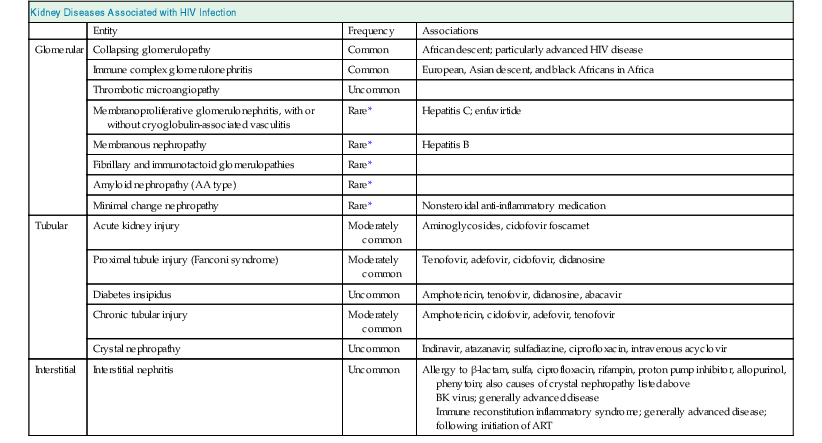
* Certain glomerular diseases are commonly associated with other viral infections or may occur on an idiopathic basis; a true association with HIV disease is uncertain or doubtful. Collapsing glomerulopathy is notable for glomerular, tubular, and interstitial disease but is classified under glomerular disease for simplicity.
Etiology and Pathogenesis
Human immunodeficiency virus–associated CG occurs with both acute infection and chronic infection; in the latter case, it is associated with higher viral RNA levels and lower CD4 T lymphocyte counts. It appears that HIV can infect glomerular and tubular cells, setting up a chronic and possibly latent infection.2 Specific HIV accessory proteins such as Vpr and Nef can damage renal cells independent of direct infection. In transgenic mice, the expression of HIV-1 accessory protein Vpr or Nef in podocytes is associated with progressive CKD, leading to ESRD, loss of podocyte differentiation markers, and features suggestive of human CG and segmental glomerulosclerosis. Transgenic mouse experiments also suggest that HIV-1 gene products are toxic to tubular epithelial cells, resulting in apoptosis and blocking cell proliferation.
Host factors also determine susceptibility to HIV-associated CG, especially in individuals of African descent when compared with those of European descent. This 20-fold predilection was originally linked with genetic polymorphisms in MYH9, encoding myosin heavy chain 9, a component of nonmuscle myosin IIA. More recently, much of the risk attributed to MYH9 genetic variation has been shown to more closely associate with coding-region polymorphisms in APOL1 (encoding apolipoprotein L1), which is in linkage disequilibrium with MYH9 polymorphisms. Apolipoprotein L1 is a constituent of high-density lipoprotein. The ApoL1 codon-changing variants confer an odds ratio of 29, a strikingly high effect for a complex disease.3 It remains unknown whether these APOL1 polymorphisms cause CKD, and by what molecular mechanism. Nevertheless, homozygosity or dual heterozygosity for two risk alleles (labeled G1 and G2) are observed in approximately 50% of African Americans with HIV-associated CG, compared with only 12% of African American controls. It is interesting to note that these variants appear to protect individuals from African sleeping sickness caused by Trypanosoma brucei rhodesiense, and this may explain the rapid rise in allele frequency in certain African populations, particularly West African populations, that has occurred during the past 40,000 years.4,5
Clinical Manifestations
Patients with HIV-associated CG typically present with laboratory abnormalities, including proteinuria and renal impairment; some patients present with edema, although edema is less common than with other conditions associated with nephrotic range proteinuria or with uremia. Imaging studies may reveal increased kidney size despite reduced glomerular filtration rate (GFR) and, in some cases, increased echogenicity; this unusual feature is shared with diabetic nephropathy and amyloid nephropathy. However, no radiologic features predict the renal histology, and renal biopsy is required for diagnosis.
Pathology
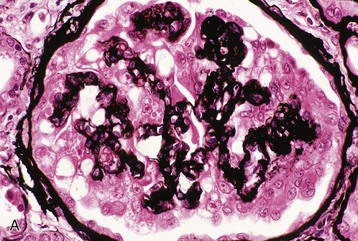
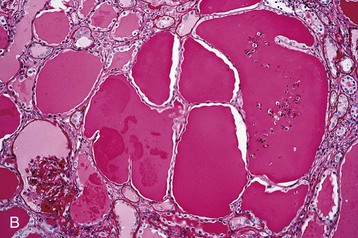
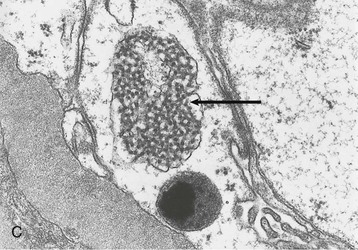
Human immunodeficiency virus–associated CG is indistinguishable from idiopathic CG, with pathologic changes in glomeruli (proliferation and dysregulation of podocytes or podocyte stem cells, together with glomerular collapse), tubules (acute and chronic injury, sometimes with microcystic tubular changes), and interstitium (chronic inflammation, fibrosis) (Fig. 58-1). Both HIV-associated CG and idiopathic CG may cause tubuloreticular inclusions within dilated endosomal compartments within glomerular endothelial cells, which is believed to be a marker for cytokine (interferon) injury. Some pathologists diagnose classic FSGS in the setting of HIV infection, and others see this rarely; it is unclear whether these differences are a result of population differences or of different diagnostic standards in distinguishing FSGS from CG. Some patients with progressive loss of kidney function (e.g., from immune complex glomerulonephritis [ICGN] or interstitial nephritis) may develop adaptive FSGS from the consequence of glomerular hyperperfusion and hyperfiltration.
Diagnosis and Differential Diagnosis
Diagnosis of HIV-associated CG is made by renal biopsy. Among individuals of African descent, nephrotic proteinuria and low CD4 count are consistent with HIV-associated CG; plasma HIV RNA of less than 400 copies/ml suggests another disease. Among individuals of African descent, other possible diagnoses include primary or adaptive FSGS (although a pathogenic role for HIV cannot be excluded in conferring susceptibility) and arterionephrosclerosis (see later). In all individuals, diabetic nephropathy and HIV-related ICGN should be considered (see later discussion and Table 58-1).
Treatment
Human immunodeficiency virus–associated nephropathy is an indication for the initiation of ART in patients who are not currently receiving these medications; this is regardless of T-lymphocyte count.6 It is reasonable to extend this recommendation to HIV-associated ICGN, despite an absence of specific evidence.
In the era before ART therapy, HIV-associated CG progressed rapidly, with patients reaching ESRD in months to a few years. The marked decline in HIV-associated ESRD in the United States after the introduction of ART in 1995 suggests that effective control of viral replication with ART may prevent HIV-associated CG. Retrospective studies also suggest that ART treatment of HIV-associated CG may prolong renal survival.7 The Development of Anti-Retroviral Therapy in Africa (DART) study, conducted in Uganda and Zimbabwe, demonstrated improvement of kidney function of 2 to 6 ml/min/1.73 m2 after 4 to 5 years of ART.8 Other studies suggest that ART is most effective when it is initiated before the onset of severe renal disease.9–12 In a few cases, regression of histologic lesions with ART has been demonstrated.12,13 The current guidelines from the Infections Disease Society of America suggest starting ART when HIVAN is diagnosed, if therapy was not used before.14 The guidelines do not address the role of ART in other renal histologies, but the authors believe it is prudent to initiate ART for any glomerular disease that is plausibly linked to HIV infection.
Treatment of HIV-associated renal disease also includes standard therapies for CKD, including control of blood pressure to below 130/80 mm Hg and the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs). Nonrandomized studies in HIV-associated renal disease suggest benefit from these interventions, but randomized controlled studies have not been performed. Several nonrandomized studies have suggested a benefit from corticosteroid therapy, but because the efficacy appears to be modest and often short-lived, this is not considered standard therapy.
Natural History
Progression of CKD is more rapid in HIV-infected individuals of African descent, probably reflecting the effect of the APOL1 variants.9 This is of particular relevance to the African population in sub-Saharan Africa that has the highest prevalence rates of HIV infection.
Patients with HIVAN may also progress to ESRD in spite of ART.15,16 Predictors of progression are a high degree of chronic damage on histology15 as well as severe renal dysfunction at baseline, high-grade proteinuria, and presence of large numbers of sclerotic glomeruli.16 Concomitant use of renin-angiotensin blockade results in improved renal survival.16
Human Immunodeficiency Virus–associated Immune Complex Glomerulonephritis
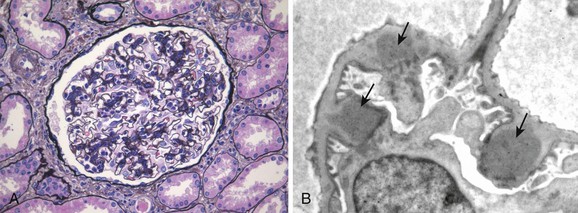
In populations of European and Asian ancestry, the most common glomerular disease associated with HIV disease is ICGN; this is true among developed countries (e.g., European Union) and developing countries (e.g., Thailand). HIV-associated ICGN is also seen in populations of African descent.17 The pathogenesis of HIV-associated ICGN is not well understood. In some cases, immune complexes include HIV antigens. Other cases may represent generalized polyclonal B-cell expansion that accompanies HIV disease. Clinically, patients typically present with nephrotic proteinuria and hematuria that are clinically indistinguishable from HIV-associated CG. Renal biopsy findings may vary from mesangial proliferative glomerulonephritis (GN) to focal or diffuse proliferative GN with endocapillary proliferation (Fig. 58-2). In some cases, changes consistent with HIV-associated CG may also be present, and tubuloreticular inclusions may be present within glomerular capillary endothelial cells. Immune deposits may be in mesangial, subendothelial, and in some cases subepithelial locations and may include IgG and IgM or IgG, IgM, and IgA (so-called “full house deposits”), often with C3. These forms resemble lupus nephritis, although lupus serologic test results are typically negative. Patients who have coinfection with HCV may manifest membranoproliferative glomerulonephritis (MPGN), with double glomerular capillary basement membrane contours. Other patients may have only mesangial IgA deposits, and this is observed especially in the setting of microhematuria and non-nephrotic proteinuria, resembling idiopathic IgA nephropathy. The long-term outcome of HIV-associated ICGN is not well defined but appears to be more favorable when histologic changes resemble IgA nephropathy as opposed to lupus-like GN. There are few studies of therapy; presumably, control of HIV infection and conservative measures to control blood pressure and proteinuria are indicated.
Other Glomerular Disorders
In addition to HIV-associated CG, FSGS, and HIV-associated immune complex disease, mentioned earlier, other glomerular disorders have been seen with HIV infection. TMA may occur with HIV infection and is associated with lower CD4 cell counts, higher viral burden, and acquired immunodeficiency syndrome (AIDS). Membranous nephropathy (MN), fibrillary GN, immunotactoid GN, and amyloidosis have also been reported in HIV infection, but a causal role for HIV infection has not been firmly established.
Arterionephrosclerosis
As HIV has become a chronic disease, extending over a period of decades, it has become apparent that chronic HIV disease is associated with premature aging, affecting various organ systems. In the kidney, this takes the form of arterionephrosclerosis, which is the constellation of arterial intimal thickening, medial hypertrophy, and duplication of the internal elastic lamina, together with global glomerulosclerosis, and is traditionally ascribed to hypertension. However, increasing evidence suggests that afferent arteriolar lesions may not only predispose to glomerulosclerosis by altering renal autoregulation, but may also have a role in the pathogenesis of hypertension. In this view, arteriosclerosis is a disorder associated with genetic mutations (e.g., APOL1), metabolic disorders (e.g., diabetes, obesity, hyperlipidemia, metabolic syndrome, and HIV infection), and chronic inflammation (smoking, oxidative stress, hemodynamic shear stress, and renin-angiotensin system activation).18 Many of these factors arise as result of lifestyles, activity patterns, diets, and habits that have been adopted throughout the world. Certain of these pathogenic factors are exacerbated in HIV infection and its therapy, including chronic inflammation, metabolic disorders, and premature aging. In a recent series of biopsies performed on HIV patients in the United States, isolated arteriosclerosis with glomerulosclerosis was the most common single pathology noted,19 and this is a trend that is likely to grow stronger.
Tubular Disorders
Human immunodeficiency virus infection is associated with several tubular disorders, many of which are secondary to drugs commonly used in this disease (see Table 58-1).20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree