HISTORY
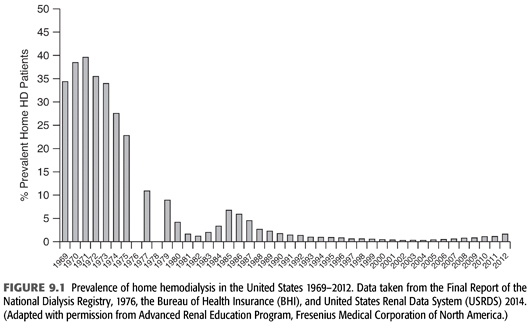
The concept of an outpatient hemodialysis center in the United States was not fully developed until the mid-1970s when legislation granted coverage for hemodialysis to most citizens regardless of age (3). This led to a rapid expansion of center-based dialysis culminating in the 2000s with thrice-weekly, center-based outpatient hemodialysis being the therapy of choice of greater than 90% of end-stage kidney disease (ESKD) patients. Prior to this event and the subsequent development of widespread, successful outpatient peritoneal dialysis in the 1980s, a large proportion of hemodialysis was performed in the patient’s home, usually by a family member or close friend (FIGURE 9.1). Some staff-assisted home hemodialysis has been done, but the associated labor costs have made this impractical for the majority of patients.
 TECHNICAL CONCERNS
TECHNICAL CONCERNS
Machine
Almost any dialysis machine can and has been utilized in the patient’s home. Several dialysis machines have been studied for safety and efficacy in the home and approved for home use by the U.S. Food and Drug Administration (TABLE 9.1) (4,5). The NxStage System One utilizes a cartridge-based extracorporeal circuit and dialysate of up to 60 L with lactate as a buffer. Its maximum dialysate flow is 300 mL/min (FIGURE 9.2). The Fresenius 2008K@home is based on a traditional hemodialysis machine requiring water treatment similar to traditional hemodialysis and bicarbonate as a buffer (FIGURE 9.3). Four other hemodialysis machines designed specifically for home hemodialysis are under development: The Tablo hemodialysis machine from Outset Medical with an integrated patient interface and production of dialysate from tap water, the PAK hemodialysis machine from Fresenius Medical, a sorbent-based hemodialysis system, the Vivia hemodialysis machine from Baxter Healthcare and the Quanta SC+ from Quanta.
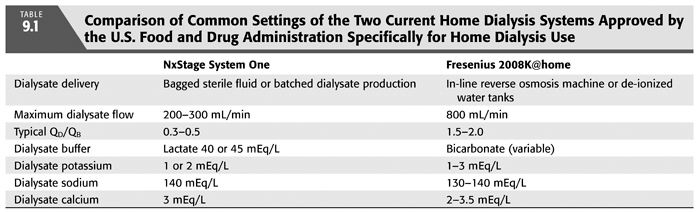


The choice of a machine is important for the home dialysis program. Machines will differ in their cost, maintenance, physical footprint, portability within and outside the home, plumbing and electrical requirements, training time, set-up and breakdown time, and water and dialysate preparation. While it is feasible and sometimes helpful to use multiple hemodialysis machines within a home program, this will introduce more required training, knowledge, and experience of the entire staff.
Water
Successful dialysis starts with the production of water free from microbiological and chemical contaminants that can harm the patient (6,7). While water production in the outpatient unit is similar in each unit, each patient’s home installation will be unique. Furthermore, while the cost of providing water and dialysate in the outpatient unit is usually less than 2% of the overall cost of the treatment, it is a major cost in the home hemodialysis treatment and also entails some cost to the patient for additional water usage and plumbing. The Centers for Medicare and Medicaid Services (CMS) has applied water quality and testing standards developed by the Association for the Advancement of Medical Instrumentation (AAMI) since 1987. These standards are updated approximately every 5 years (8). The water quality of both the outpatient unit and the patient’s home is ultimately the responsibility of the medical director of the dialysis facility. These general principles of water treatment for hemodialysis are covered in Chapter 8.
In the home environment, the water system will be unique in every installation while adhering to the same AAMI standards. The source water should be tested to see if it meets the minimum standards of the Safe Water Drinking Act (9). If the source water does not meet these standards, then the product water should undergo an AAMI analysis to ensure the contaminants have been removed by the water treatment system. Numerous water supplies in the United States have elevated levels in the source water of elements such as iron, manganese, sulfates, copper, lead, aluminum, and others that will need to be tested in this way. Furthermore, occasional contamination of municipal water supplies such as the Elk River accident with 4-methyl cyclohexane methanol in West Virginia in 2014 can endanger the home hemodialysis patient (10). Most water treatment systems will adequately remove these contaminants; however, the poor source water quality may lead to early exhaustion of components of the treatment system at substantial cost and inconvenience. In this circumstance, installation of a second system between the source water and the water treatment system will often alleviate the problem.
Dialysate
Dialysate can be provided in a patient’s home in a number of methods: bagged dialysate delivered to the patient’s home similar to peritoneal dialysis, dialysate prepared in the patient’s home prior to dialysis, or in-line dialysate from an appropriate water source mixed at the machine. Cost, convenience, storage, installation, and maintenance all factor in the type of dialysate to utilize.
Bagged dialysate similar to peritoneal dialysis has the advantage of providing a sterile, ultrapure dialysate to the patient. However, limitations accompany this advantage. Practical considerations of delivery, storage, cost, and logistics generally limit this method to approximately 30 L of dialysate yielding a single pool urea Kt/V of approximately 0.7 in the typical 80-kg adult; thus, in the absence of significant residual renal function, more than three times a week dialysis will be needed to obtain what is currently considered “adequate” hemodialysis based on small molecule clearance. Second, lactate is typically the base in bagged dialysate fluid for both stability and microbiological concerns. Lactate showed improvement in tolerability over acetate as a hemodialysis buffer before the widespread introduction of bicarbonate-based buffer (11). Yet, in hemodialysis with a lactate buffer, serum lactate levels will be increased slightly, but patients with significant liver dysfunction, higher volumes of dialysate, and/or poorly controlled diabetes may not tolerate lactate as defined by a serum lactate level of >5 mEq/L and transient symptoms of muscle cramping, lower blood pressure, nausea, headache, or weakness. Lastly, the production of bagged dialysate offers a limited number of dialysate baths negating one of the benefits of home hemodialysis—optimizing the dialysis prescription for the individual.
Replicating dialysate production similar to the outpatient unit or the acute care setting in the hospital also has limitations. Typically, either a reverse osmosis (RO) machine or a deionized (DI) water system must be installed in the patient’s home. The RO machine adds another machine to install, maintain, and monitor. The DI system usually requires an outside vendor to change the tanks and regenerate the beads in addition to plumbing installation delivering the water to the machine in the home.
Several systems that produce water and dialysate in novel methods in the home are being developed. These include the use of sorbent, distillation, and miniaturization of the dialysate production.
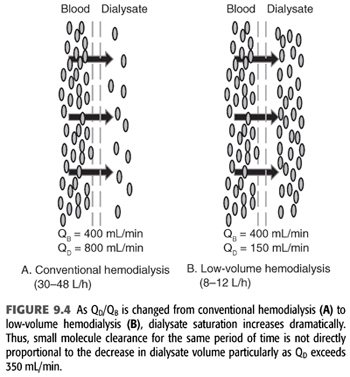
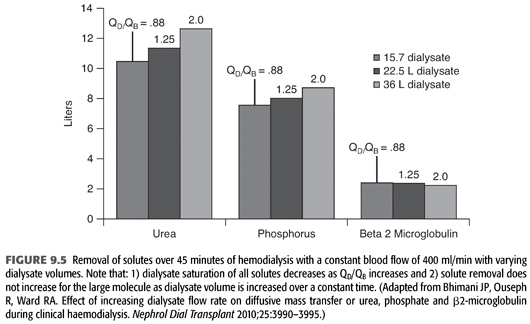
Home dialysis regimens often use lower dialysate flows (<500 mL/min) and total dialysate volumes (<60 L) necessitating a change in standard chemical components of the dialysate bath, particularly small molecules such as potassium (12). Some have termed this low-volume dialysis, but no consistent term has been applied (FIGURE 9.4). Whereas a conventional hemodialysis treatment may use a blood flow of 400 mL/min and a dialysate flow of 600 mL/min, home dialysis treatments may use a dialysate flow much lower such as 200 mL/min. As dialysate flow is decreased relative to blood flow, the dialysate effluent will be more highly saturated (FIGURE 9.5). To remove the same amount of solute over the same time, the starting gradient in the dialyzer must be higher (13,14). For example, a potassium bath of 2 mEq/L for a 3-hour conventional, high-efficiency hemodialysis treatment with a dialysate flow of 600 mL/min (108 total L of dialysate with approximately 20% solute saturation) will need to be changed to 1 mEq/L for the 3-hour home hemodialysis treatment at 200 mL/min (36 total L of dialysate with approximately 80% solute saturation).
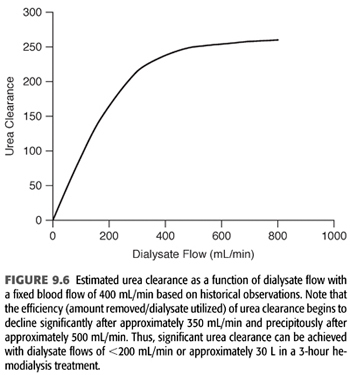
Finally, it is important to note that as dialysate flow is increased relative to blood flow, the incremental amount of small molecule clearance rapidly decreases particularly as dialysate flow moves above approximately 350 mL/min (FIGURE 9.6). Because of the incremental cost of additional water and dialysate preparation, several dialysis providers have reduced their conventional dialysate flow to a maximum of 500 mL/min and suggested even 400 mL/min.
One of the more common home dialysis machines, the NxStage System One, utilizes a fixed dialysate flow to blood flow ratio (“the flow fraction” or QD/QB), typically less than 0.5, in order to saturate the relatively small amount of dialysate. Adjusting the “flow fraction” down will increase the saturation of the dialysate or the efficiency of the dialysis treatment, while increasing the flow fraction will decrease the efficiency of the treatment. Similarly, the Fresenius 2008K@home machine can utilize a fixed QD/QB of 1.5 or 2.0 (“autoflow”).
 CLINICAL CONCERNS
CLINICAL CONCERNS
Patient Selection
Not every patient is willing or capable to perform hemodialysis at home. Placing a patient who is unwilling to follow treatment protocols at home raises additional safety concerns. Furthermore, training patients for home hemodialysis is a time-consuming, labor-intensive, and expensive process. If patients fail at home shortly after training, both nursing morale and the financial performance of the home dialysis program suffer.
However, choosing patients whom in some physician’s eyes seem to be the perfect candidate for home therapy—less comorbid illnesses, employed, extensive social support networks, younger age, well-educated, and so on—does not necessarily ensure success. First, that “ideal” candidate rarely exists. Second, many of those patients are doing well with their current therapy and may not see a substantial improvement with home therapy. Lastly, the patient whom may benefit the most from home hemodialysis and succeed at home hemodialysis is not well-defined and not always obvious to the physician.
In lieu of studies or trials that evaluate this question, a simplified approach generally works. First, why does the patient want to perform home hemodialysis or, alternatively, why is the physician recommending home hemodialysis? Is the specified expectation or reason a measurable outcome that can be evaluated in a reasonable time frame (TABLE 9.2). Second, what type of support at home does the patient have? Support should not be confused with effort by family members, friends, or care partners in the home. Patients should be encouraged to perform as much of the dialysis treatment as possible and be involved in their care as much as possible. Kidney failure and dialysis is a long and difficult journey, and encouragement and support are critical to the success, particularly at home. Having a spouse, family member, or friend actually performing the dialysis treatment is not crucial to success for every patient. In fact, the additional stress on the “care partner” may lead to modality failure despite the improvement of the patient clinically. Lastly, while it seems that the amount of support would correlate with success at home, some data suggests that success is more likely to occur where the patient’s expectation of support matches what is actually received rather than the absolute quantity delivered (15).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


