Fig. 2.1
Normal distal esophagus, gastroesophageal (GE) junction, and stomach after formalin fixation. The squamous mucosa is pinkish white, the GE junction is well demarcated, and the stomach shows normal darker pink columnar mucosa with rugae
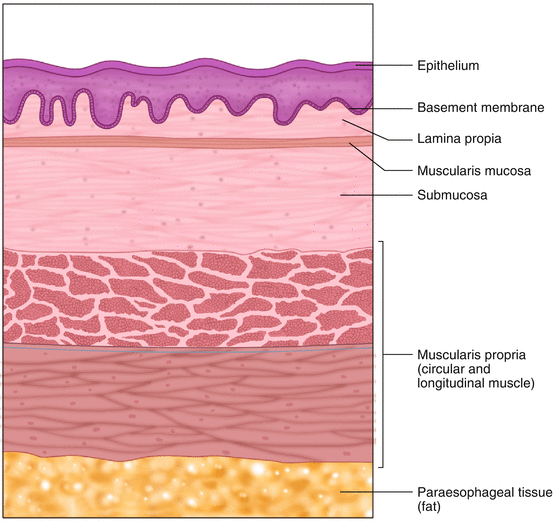
Fig. 2.2
Schematic view of a normal full-thickness section of esophagus
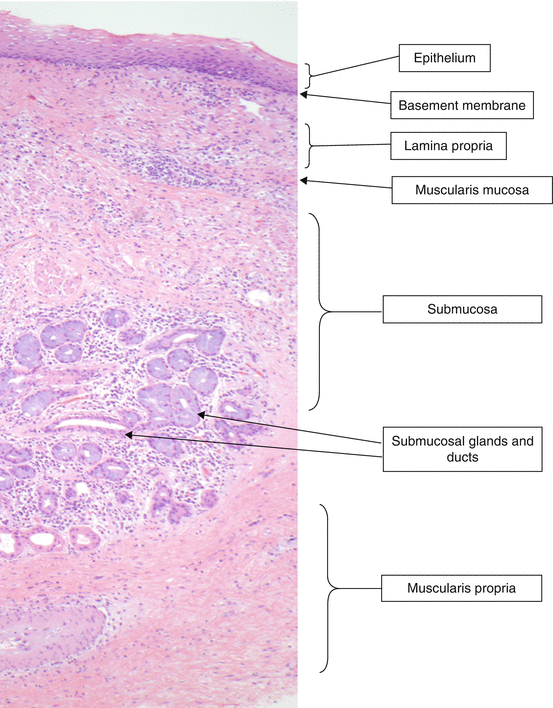
Fig. 2.3
Normal histology of a full-thickness section of esophagus (hematoxylin-eosin [H & E], ×20)
Esophagitis
Changes of reflux esophagitis include thickening/proliferation of the epithelial basal cell layer with dilated intracellular spaces, elongated lamina propria papillae, and intraepithelial eosinophils (Fig. 2.4a).
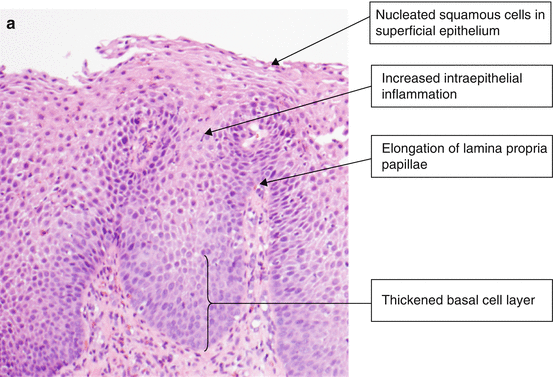
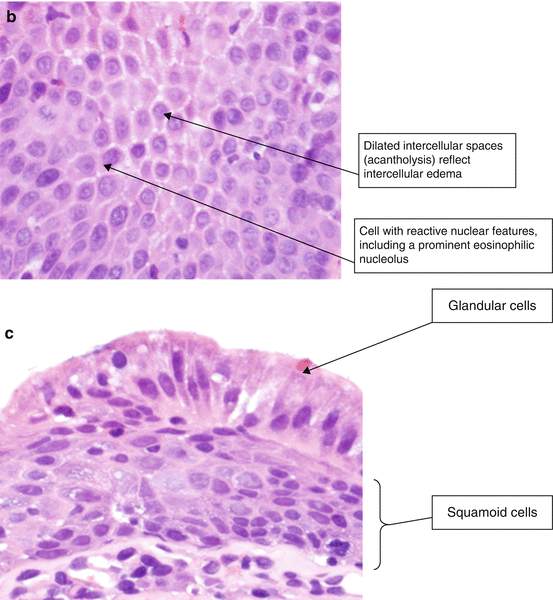


Fig. 2.4
Histology of esophagitis. (a) Reflux esophagitis (H & E, ×100). (b) Reflux esophagitis, basal layer of epithelium (H & E, ×400). (c) Gastroesophageal junction with multilayered epithelium (H & E, ×400)
Increased lymphocytes may be seen in the epithelium and lamina propria (Fig. 2.4b) [2]. Note the dilated intercellular spaces (spongiosis), reflective of intercellular edema. The nuclei have reactive changes including enlargement and prominent nucleoli with retention of smooth nuclear and nucleolar contours.
Multilayered epithelium (Fig. 2.4c) is characterized by the presence of columnar glandular cells overlying squamous cells. This change is associated with reflux esophagitis, but also may be seen in biopsy specimens taken from patients who go on to develop Barrett’s esophagus, as reflux is often seen in these patients as well [3].
Barrett’s Esophagus
The squamocolumnar junction is composed of squamous epithelium and glandular epithelium (Figs. 2.5 and 2.6a). In the United States, Barrett’s metaplasia is defined by the presence of columnar epithelium with goblet cells. The metaplastic epithelium must be located in the tubular esophagus. The goblet cells are distended by blue-hued acid mucin. Note that the epithelium is mature at the surface, as the cells have abundant cytoplasm and small nuclei that are oriented toward the basement membrane. No dysplasia is seen Fig. 2.6a.
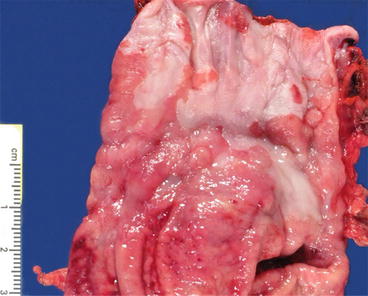
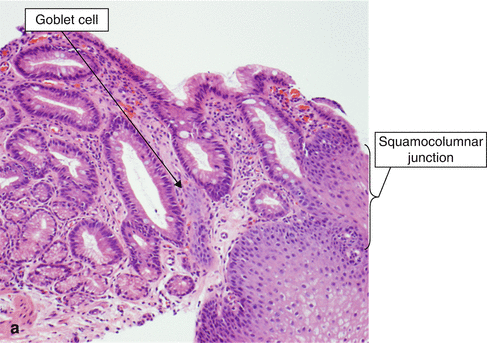
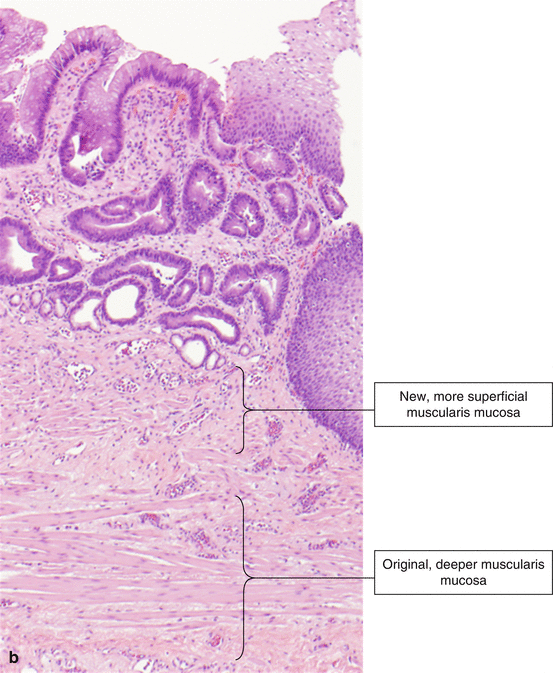

Fig. 2.5
Gross photo of gastroesophageal junction with Barrett’s esophagus (fresh specimen)


Fig. 2.6
Barrett’s esophagus, histology. (a) Barrett’s esophagus (H & E, ×100). (b) Barrett’s esophagus (H & E, ×40)
Another feature of Barrett’s esophagus is duplication of the muscularis mucosa (Fig. 2.6b). The original muscularis mucosa is located deeper than the new layer that is associated with Barrett’s esophagus. The new layer is closer to the lumen of the esophagus and is composed of thinner, frayed smooth muscle fibers. There may be edematous lamina propria between the two layers.
A diagnosis of Barrett’s esophagus indefinite for dysplasia may be rendered under several circumstances. In Fig. 2.7a, for example, glands lined by atypical, metaplastic epithelium are seen beneath squamous epithelium; they are “buried.” This neoepithelialization may occur after radiofrequency ablation treatment of Barrett’s esophagus. The glandular cells have nuclear hyperchromasia and enlargement, some stratification, and increased mitotic activity. The degree of surface maturation of the glandular epithelium cannot be assessed in this sample because of the overgrowth of squamous epithelium. As a definitive diagnosis of dysplasia cannot be rendered, the diagnosis of “indefinite for dysplasia” is felt most appropriate. However, the mere presence of buried metaplastic epithelium is insufficient to warrant a diagnosis of indefinite for dysplasia.
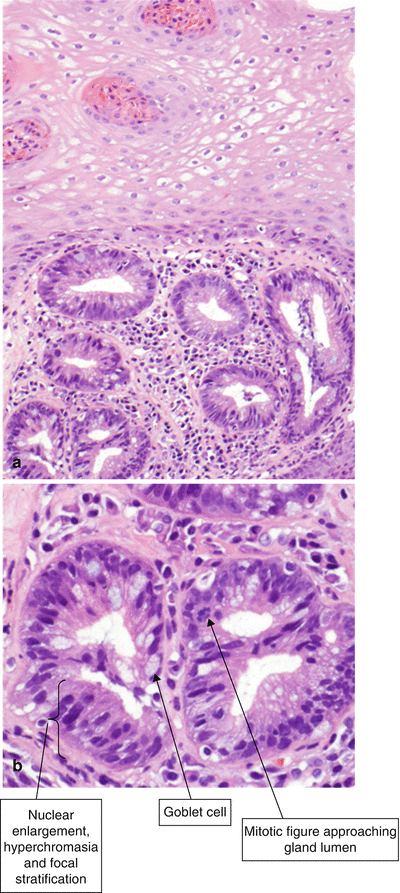

Fig. 2.7
(a) Barrett’s esophagus, indefinite for dysplasia (H & E, ×100). (b) Barrett’s esophagus, indefinite for dysplasia (H & E, ×400). The cells show cytologic features of dysplasia that include nuclear enlargement, hyperchromasia, and some nuclear stratification. Diagnostic features of high-grade dysplasia are not seen
Other situations that may warrant a diagnosis of indefinite for dysplasia include: (1) the presence of marked reactive atypia in the setting of ulceration or inflammation; (2) atypia limited to the bases of the glands (not extending to involve the surface epithelium); (3) tangential sectioning, absence of surface epithelium, or other artifactual changes; and (4) cytologic and architectural changes that are worrisome for, but not diagnostic of, dysplasia (Fig. 2.7b) [3].
Barrett’s Esophagus, Low-Grade Dysplasia
Low-grade dysplasia is characterized by nuclear crowding, stratification, atypia, and increased mitotic figures (Fig. 2.8). The atypical nuclei extend beyond the crypt epithelium to involve the superficial epithelium. Focal goblet-cell depletion may also occur. The contours of the dysplastic glands are more irregular than those without dysplasia. In contrast to high-grade dysplasia, low-grade dysplasia retains some nuclear polarity (most nuclei are oriented toward the basal aspect of the cell, away from the luminal surface) and the nuclei remain elongated or cigar-shaped.
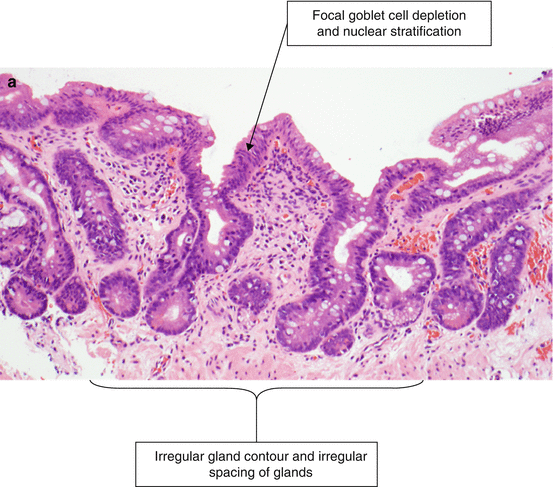
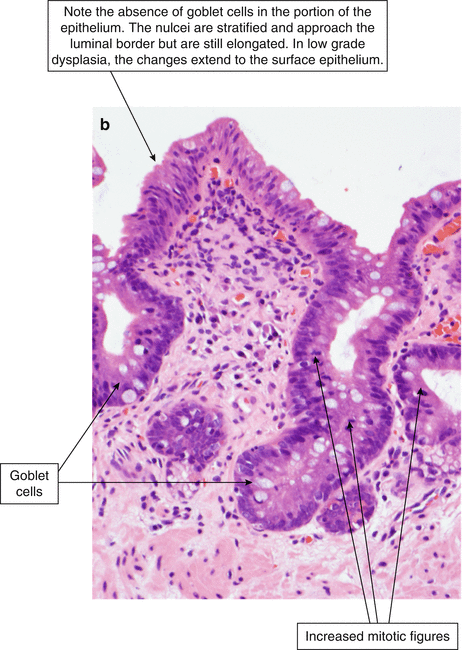


Fig. 2.8
(a) Barrett’s esophagus, low-grade dysplasia (H & E, ×100). (b) Barrett’s esophagus, low grade dysplasia (H & E, ×200). Review of the histological specimen by two histopathologists is recommended to diagnose and characterize dysplasia in Barrett’s esophagus
Barrett’s Esophagus, High-Grade Dysplasia
High-grade dysplasia shows more marked nuclear atypia, which extends to the superficial epithelium (Fig. 2.9). The nuclei are hyperchromatic, enlarged, and rounded, often with irregular nuclear contours. There is loss of nuclear polarity, often with full-thickness nuclear stratification. Increased mitotic figures (including atypical forms) are seen, and goblet cells are notably decreased. Some features, when present in biopsy specimens showing Barrett’s esophagus with high-grade dysplasia, are predictive of invasive carcinoma upon surgical resection. These features include gland cribriforming, dilated glands with necrotic material, ulceration of high-grade dysplasia, invasion of dysplastic glands into the overlying squamous epithelium and the presence of many neutrophils within the epithelium with high grade dysplasia [4].
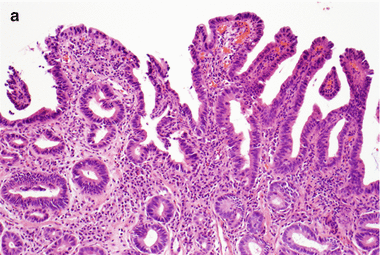
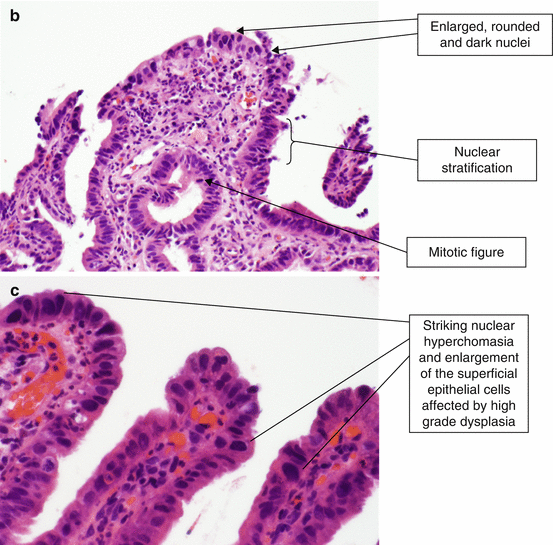


Fig. 2.9
(a) Barrett’s esophagus, high-grade dysplasia (H & E, ×100). (b) Barrett’s esophagus, high-grade dysplasia (H & E, ×200). (c) Barrett’s esophagus, high-grade dysplasia (H & E, ×400)
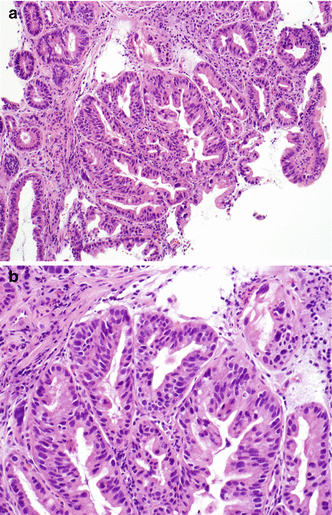
Adenocarcinoma
The presence of dysplastic glands beyond the epithelial basement membrane and within the lamina propria or duplicated muscularis mucosa defines intramucosal adenocarcinoma (Fig. 2.10). Invasion through the true muscularis mucosa and into the submucosa is not identified. The nuclear morphology is similar to that seen in high-grade dysplasia. The gland architecture is complex and crowded, with back-to-back glands lacking intervening stroma. Angular gland contours, intraluminal necrosis, and dilatation of gland lumina may also be seen. In addition, single malignant cells may be present within the lamina propria. As lymphatics are present in the lamina propria of the esophagus, there is a small risk of metastasis [5]. The histological diagnosis of intramucosal adenocarcinoma corresponds to T1a disease, with invasion into the lamina propria or muscularis mucosa. This finding is important, as the 15–20 % rate of lymph node metastases with submucosal tumors (T1b disease) is higher than the rate for T1a disease (5–7 %).