Fig. 45.1
Sonablate® device
Fig. 45.2
Ablatherm® device (Courtesy of EDAP)
Mechanisms of Action
In contrast to its use in ultrasound imaging techniques, the high acoustic energy leads to higher temperatures enough to cause a coagulation necrosis when focused on a precise tissue point [4]. In initial stages there is generation of microbubbles with the absorption of the energy and heat generation. The interaction between microbubbles and ultrasounds produces a cavitation effect resulting in cellular, and subsequent tissue destruction. Both thermal and cavitation effects are responsible of the tissue destruction by coagulative necrosis [5]. The sum of elementary lesions applied tight to each other allows a volume targeting compatible with prostate gland shape destruction.
The two available devices – Sonablate® (Focus Surgery Inc, Indianapolis, Ind, USA) and Ablatherm® (EDAP-TMS SA, Lyon, France) deliver focused ultrasound through a transrectal approach. The basic working principles are same apart from some technical differences. A transrectal high-frequency transducer in a balloon filled with water to prevent heating of the rectal wall (thereby minimizing the risk of recto-urethral fistula) is placed in the rectum. There is also a mechanism to monitor rectal temperatures.
Sonablate® offers transducers of different focal lengths (25–45 mm) with a fixed elementary lesion length of 10 mm × 2 mm in width while Ablatherm® includes a unique focal energy transducer (40 mm) and an imaging transducer in the same endorectal probe, thus allowing a real-time control of imaging the treatment (Fig. 45.3); elementary lesion length varies from 19 to 26 mm × 2 mm in width (Figs. 45.4, 45.5, and 45.6). Generally speaking for the two devices, the volume of the prostate at the time of delivery of HIFU has to be less than 35 cc. Therefore, a TURP or even a previous adenomyomectomy in high volume prostates may be beneficial to achieve an adequate volume at the time of HIFU. In fact there is evidence to show that a previous TURP before HIFU, reduces the chances of acute urinary retention and bladder outlet obstruction after HIFU treatment. This can potentially reduce the time of urethral catheterization (4 days vs. 15 days) [6–9].
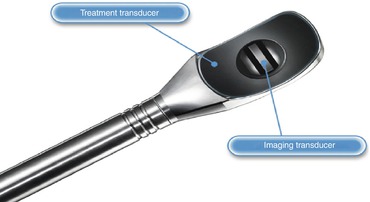
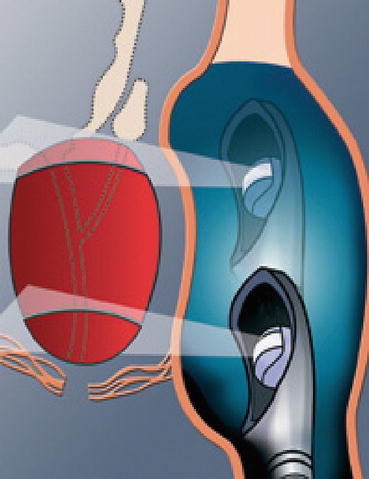
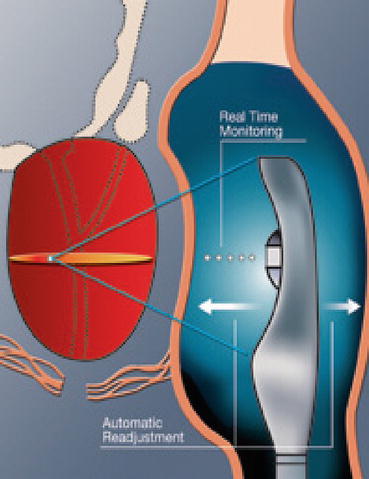
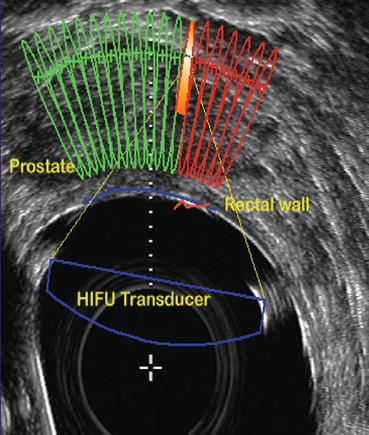

Fig. 45.3
Imaging and treatment transducer (Ablatherm® device) (Courtesy of EDAP)

Fig. 45.4
Ultrasound probe used for imaging (Courtesy of EDAP)

Fig. 45.5
delivering HIFU (Courtesy of EDAP)

Fig. 45.6
Real time control imaging (Courtesy of EDAP)
Indications for HIFU
It is important to note that most of the available data has been retrospective and long term results and potential use of HIFU as a primary treatment similar to radical prostatectomy or EBRT need to be confirmed by randomised trials. There is also lack of consensus on various PSA thresholds and objective response criteria.
Various guidelines including European Association of Urology (EAU), the American Urologic Association (AUA), the UK National Health Service based National Institute for Health and Clinical Excellence (NICE) Prostate Cancer guidelines and the US Federal Drug Administration do not currently recommend HIFU as a standard treatment for the management of clinically localized prostate cancer [10–12].
However, the management of PCa by HIFU could be considered in three settings:
(a)
As a primary treatment for localised prostate cancer (T1c-T2a, N0 M0)
(b)
Salvage therapy after failure of EBRT or Brachytherapy
(c)
Focal HIFU therapy
Contraindications for HIFU
There are some relative contraindications for HIFU but a rectal thickness >6 mm or rectal stenosis are the true real contra-indications of an HIFU treatment. In patients with of chronic inflammatory bowel disease the choice of treatment of PCa could be challenging and HIFU treatment is a feasible when employed cautiously [13]. As mentioned earlier gland volume is a relative contraindication. Any interference with ultrasound imaging such as prostatic stones can interfere with the procedure. This could be avoided by doing a TURP prior to the procedure.
HIFU as a Primary Care Treatment
According to the French Urological Association, HIFU can be an option as a primary care treatment for the specific patients (Table 45.1)
Table 45.1
Criteria defined by French Urological Association
Age >70 years and >7 years of life expectancy |
Clinical stage T1 or T2 |
PSA <15 ng/ml |
Gleason ≤7 |
Prostate volume <50 cc |
HIFU can be repeated several times – It has been shown that a second HIFU session may improve oncological control [14–16]. However, there is no gain beyond two HIFU sessions and on the contrary there is a possibility of increase in morbidity [17, 18].
Salvage HIFU After EBRT Failure
Considering the high rate of positive biopsies after EBRT of 30–40 % [19] and the significant morbidity involved in salvage prostatectomy [20–23], the role of HIFU as a salvage option has been addressed since 1993 in specialized centres like Lyon University Hospital [24]. The key points in selecting these patients are, to confirm the local recurrence by prostate biopsies, exclude any detectable distant metastasis (with whole body CT scan, bone scintigraphy and eventually with [10] Choline PET/CT). An assessment is then made regarding the benefit of the treatment with a curative intent (considering the predictive factors of success) against the side effects of HIFU in this setting (see results section). In relation to brachytherapy failure, clinical trials are still ongoing with a very few published data at present [25].
Focal HIFU Treatment
The European Randomised Study of Prostate Cancer (ERSPC) has concluded that there is a reduction of mortality of prostate cancer thanks to screening of PCa but the screening has an underlying risk of overdiagnosis and therefore overtreatment [26]. Similarly, Cooperberg et al. have shown that selected men with intermediate risk features active surveillance may be appropriate as in these men the cancer is not likely to progress [27]. Although active surveillance is gaining a growing interest, it is worth noting that between 20 and 30 % of patients are misclassified and nearly 30 % of these patients will ultimately need a radical treatment. The negative impact of active surveillance in terms of progression of the cancer has still to be addressed [28, 29]. Nearly 20 % of PCa are located on one side only according to a series of radical prostatectomies [30]. In such patients, to prevent overtreatment and undertreatment focal therapy may be the choice. The ideal conditions to for focal treatment are: that the treatment should be feasible (focal destruction proven), there has to be endpoints to address efficacy of treatment, treatment should be cost-effective, feasibility of another type of radical treatment in case of failure. All these criteria must be satisfied before undertaking the clinical trials. Although there are no major safety concerns about the treatment, evidence acquisition regarding mechanism of action and side effects is still lacking.
HIFU Outcomes
HIFU as a Primary Care Treatment
Oncological outcomes: Table 45.2 show results of HIFU as a primary treatment for the major series. As mentioned earlier, one of the limitations to address HIFU results properly is the relative heterogeneity of endpoints. Many series have used Phoenix definition to assess the biochemical failure. One of the main criticisms of usage of Phoenix definition is that it has been exclusively for radiation and not for other physical agents [34].
Table 45.2
Results of primary HIFU treatment
Author | Institution (device S/A) | Year | No of patients | Median/mean follow-up (months) | Negative biopsy rate | Definition of success | 5 year BFSR | 5 year DFSR |
|---|---|---|---|---|---|---|---|---|
Uchida et al. [31] | Tokyo (S) | 2009 | 517 | 24 (median) | – | Phoenix | 72 % | – |
Ahmed et al. [32] | London (S) | 2009 | 172 | 12 (mean) | – | PSA nadir ≤0.5 | 78 % | |
Psa nadir ≤0.2 | 58 % | |||||||
NEDa | 92 % | |||||||
Blana et al. [33] | Regensburg/Lyon (A) | 2008 | 140 | 76 (mean) | 86 % | Phoenix | 77 % | 66 %b |
Thuroff et al. [15] | European multicentric (A) | 2003 | 402 | 13 (mean) | 87 % | – | – | – |
Crouzet et al. [14] | French multicentric | 2010 | 803 | 42 (mean) | 85 % | Phoenix | 83 % LR | 72 % LRc |
72 % IR | 56 % IR | |||||||
68 % HR | 47 % HR |
Usually PSA nadir is achieved around 3 months after HIFU. As the nadir value has a strong predictive value with a threshold of 0.2 and 0.5 ng/ml [35, 36], it is used as a criteria to evaluate the results. This can also be supplemented with systematic biopsies if it is deemed that nadir PSA values are insufficient. Early post HIFU evaluation with positive biopsies gives at least three options with a curative intent: treatment with a second HIFU session, salvage radiotherapy (as it has shown excellent oncological control after HIFU) and finally even radical prostatectomy after HIFU is a feasible option [18, 37–39]. All these endpoints taken together, the efficacy of HIFU can be evaluated through its biochemical results, or through an ”adjuvant treatment free survival rate” since the decision of an additional treatment clearly represents a failure of HIFU treatment. Irrespective of the type of device used, HIFU achieves a biochemical control of prostate cancer in 58–83 % patients depending on the risk group and the adopted definition. Disease free survival rates range from 47 to 72 % according to high, intermediate, low-risk group disease at a median follow-up of 42 months [14, 31–33].
Functional Outcomes
Due to the effects of tissue destruction and high temperature effects in the prostate, patients can encounter voiding problems after HIFU, either due to outlet obstruction or urinary incontinence. Obstruction can be due to a urethral stenosis and/or a bladder outlet obstruction: these symptoms may be observed 3–12 months after the procedure and are reported be seen in 3–15 % of cases. These symptoms require endoscopic intervention in 3–10 % of cases [8, 40]. The rate of urinary leakage is reported to be between 0.5 and 22.5 % [17, 41–43]. In most cases it resolves within 1 year. Generally speaking, in most series significant grade 3 urinary incontinence mentioned is ≤5 %. Potency after HIFU has been prospectively addressed in a quality of life survey on 326 patients, showing that 52–78 % of patients remained potent after HIFU with gradual improvement in a 24 months-period [44].
Salvage HIFU After EBRT Failure
Oncological Outcomes
Table 45.3 summarizes the results of oncological outcomes in patients who were treated with HIFU after failure of radiotherapy treatment. With the Sonablate® device, Uchida et al. described a 52 % biochemical control rate while Zacharakis et al. reported no evidence of disease in 71 % of their patients in their series; also half of their patients achieved a PSA level of <0.2 ng/ml [25, 45].
Table 45.3
Results of salvage HIFU
Author | Institution (device S/A) | Year | No of patients | Median/mean follow-up (months) | Neg. biopsy rate | Median nadir PSA | 5 year BFS | 5 year DFSR |
|---|---|---|---|---|---|---|---|---|
Uchida et al. [25] | Tokyo (S) | 2011 | 22 | 24 (median) | 91 %a | LR 100 % | – | |
IR 86 % | ||||||||
HR 14 % | ||||||||
Zacharakis et al. [45] | London (S) | 2008 | 31 | 7 (mean) | – | 71 % NED | ||
Murat et al. [18] | Lyon (A) | 2009 | 167 | 18 (mean) | 73 %b | 0.19 | LR 53 % | |
IR 42 % < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




