Hepatic Resection: General Considerations
Jean-Nicolas Vauthey
Junichi Shindoh
Introduction
Resection is the first-line treatment in selected patients with primary or metastatic hepatic malignancies. In recent decades, refinements in surgical techniques and in perioperative patient care have improved the safety of liver resection; however, the most important factor influencing outcomes after liver resection is the surgeon’s knowledge of the basic surgical principles pertaining to the procedure. Postoperative morbidity and mortality rates can be reduced by proper patient selection, attention to liver anatomy and volumetry, and use of the optimal approach and technique for resection. At largevolume centers, the 90-day mortality rates after liver resection are now less than 5%, and the rate of complete resections with negative margins is approaching 90%. These rates are not likely to be substantially further improved, especially as the limits of resectability are continually being pushed; therefore low morbidity rates and early recovery will have to be considered as the new primary endpoints. In this chapter, we report the general principles pertaining to the safe and complete resection of liver tumors.
Preoperative Assessment
In recent years, the eligibility criteria for liver resection have been expanded to include patients not previously deemed to be surgical candidates, such as those with multiple bilobar liver metastases from colorectal cancer and those with large or multinodular hepatocellular carcinoma (HCC). However, the current definition of resectability still requires that the surgeon be able to completely remove the tumor while preserving a sufficient remnant of healthy liver tissue to limit the risk of postoperative liver dysfunction. This oncosurgical definition necessitates attention to (1) the extent of the tumor and (2) the quality and volume of the anticipated remnant liver after negative margins are achieved.
Evaluating Tumor Extent
In recent years, advances in imaging technology have made the preoperative evaluation of liver tumors more precise, contributing to both the improvement and safety of liver resection.
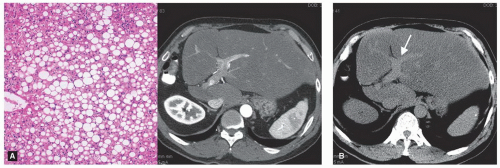
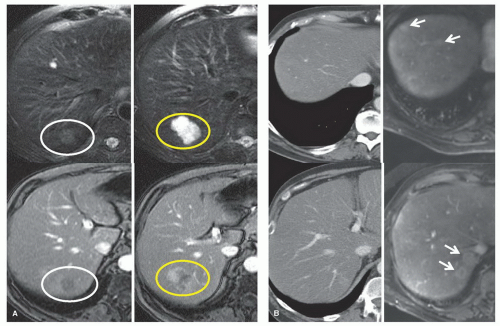
Helical computed tomography (CT) with a liver protocol (quadruple phase with rapid injection of 150 ml of intravenous contrast material and slice thickness of 2.5 to 5.0 mm through the liver) can accurately evaluate the extent of the tumor or tumors in the liver and each tumor’s relationship with the biliary tract and vascular structures. Three-dimensional reconstruction of CT images can be used to better assess the liver’s segmental anatomy and volumetry. Chest CT has replaced chest x-ray as the preferred modality for identifying lung metastases in patients with liver tumors. The routine use of enhanced magnetic resonance imaging (MRI) has generally not been recommended because MRI has not been demonstrated to be more accurate than CT for most patients and because it is less reliable for detecting extrahepatic disease, particularly in the chest or peritoneum. However, MRI should be performed for further characterization of presumably benign or atypical liver tumors or when the contrast agents used for CT are contraindicated. In addition, new MRI contrast agents are potentially very useful for delineating hepatic disease extent, particularly in the setting of hepatic steatosis (Figs. 18.1, 18.2).
Because of the improvements in image resolution mentioned above, laparoscopy is less frequently indicated to assess the extent of liver tumors, although additional hepatic disease may well be identified and is still used in selected patients to evaluate for extrahepatic disease, chronic liver disease, or hepatic injury associated with extended chemotherapy.
Although recommended by some surgeons as part of preoperative evaluation, positron emission tomography (PET) is not used routinely for primary liver cancer or liver metastases at all centers. Importantly, PET-CT should not replace high-quality CT imaging combined with interpretation by a radiologist with hepatobiliary expertise. PET-CT is not useful in patients who have received preoperative chemotherapy for colorectal cancer liver metastases because the response to chemotherapy is associated with decreased PET sensitivity.
Evaluating Determinants of Postoperative Liver Function
Liver function after liver resection depends on the quality of the liver parenchyma, the volume of the future liver remnant (FLR), and the regenerative capacity of the liver. The risk of postoperative liver failure remains high after major or extended liver resection. This risk should be estimated preoperatively to determine whether resection is safe and to optimize the postoperative outcome.
In patients with chronic liver disease, the functional reserve of the liver is assessed using composite scoring systems that include biologic data, such as the Child-Pugh classification system for liver disease (Table 18.1). Usually, only patients with Child-Pugh class A disease are considered eligible for liver resection because postoperative mortality rates are higher for patients with higher Child-Pugh class, approaching 50% for those with Child-Pugh class C disease. Since the presence of undiagnosed subclinical portal hypertension can considerably increase the risk associated with surgery, patients should be screened preoperatively for clinical signs of portal hypertension (for ascites, collateral venous circulation), biologic assessment (for platelet count <100,000) and imaging (for evidence of venous collaterals or splenomegaly).
TABLE 18.1 Child-Pugh Score for Hepatic Functional Reserve | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
A biopsy of the nontumorous liver parenchyma can be used to evaluate for evidence of underlying liver disease. However, this approach has two main limitations. First, the distribution of liver diseases such as fibrosis, steatosis, or chemotherapy-associated liver injury is heterogeneous and the severity of chronic liver disease may not be accurately assessed. Second, histopathologic findings do not accurately reflect liver function and the regenerative capacity of the liver that is pivotal for liver regeneration after major resection.
Indocyanine green (ICG) clearance can also be used to assess liver function. The ICG retention rate at 15 minutes has been adopted in Asia to evaluate liver function in patients with chronic liver disease before resection. However, ICG R15 is a test of global liver function, and it is valuable after minor (limited) resection but may not be as useful as in patients undergoing major resection.
Evaluating the FLR’s volume is currently the most reliable approach to predict outcomes for patients who are candidates for major liver resection. Several methods for such evaluation have been described. At The University of Texas M. D. Anderson Cancer Center, we calculate the estimated total liver volume (TLV) using a formula that relies on the linear correlation between the TLV and body surface area (BSA): TLV (in cm3) = -794.41 + 1,267.28 × BSA (in m2). The standardized FLR is then calculated as the ratio of the FLR volume to the estimated TLV. Therefore, the standardized FLR is the FLR as a percentage of the TLV estimated using the above mentioned formula. In a series of 301 patients without chronic liver disease or hepatic injury undergoing extended right hepatectomy for liver tumors, we found that a standardized FLR of equal to or less than 20% was a risk factor for postoperative liver insufficiency and 90-day postoperative mortality.
In patients with small FLRs, portal vein embolization (PVE) can be used to promote hypertrophy of the FLR, making curative resection possible for a subset of patients previously deemed to have borderline or unresectable disease. PVE is recommended for resection that would leave a remnant liver less than or equal to 20% in patients with normal liver, less than or equal to 30% in patients with hepatic injury, such as those who have received extensive chemotherapy (>3 months), and for resection leaving a remnant liver less than or equal to 40% in patients with fibrosis or cirrhosis (Fig. 18.3). PVE is usually performed under fluoroscopic guidance and involves the cannulation of the ipsilateral branch of the portal vein and the embolization, using microparticles followed by coils or absolute ethanol, of the entire portal vein tree to be resected (Fig. 18.4




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree