Fig. 8.1
Liver parenchyma using cavitron ultrasonic surgical aspirator (CUSA). The liver parenchyma is fragmented by ultrasonic energy and simultaneously aspirated, leaving the vascular and ductal structures to be ligated or sealed by electrocautery
8.4.3 The Water Jet Dissectors
In the 1980s, the water jet (hydrojet) dissectors appeared for liver resection. The technique was first reported to be applied in 45 lobectomies in dogs and in 4 liver resections in humans [23]. The water jet dissector employs a pressurized jet of water to fragment the liver parenchyma tissue and leave the vascular and ductal structures visible and easily controlled during dissection (Fig. 8.2). Rau and colleagues refined this technique in in vitro and in vivo trials and introduced it into clinical routine in liver surgery [24]. Rau et al. found that a pressure of 30–40 bar and a nozzle diameter of 0.1 mm are very effective to dissect normal liver tissue, and the pressure needed for dissection is 10 bar higher in case of cirrhotic liver parenchyma. However, one disadvantage of water jet and CUSA in liver transection is the long transection time because of the need for ligation or clipping of individual vessels. There are also concerns of increased risk of venous air embolism with water jet technique, although this appears to be a clinically rare problem [25, 26]. In the practice, the water jet should be used in the cirrhotic liver with more experienced, a higher jet pressure is needed to cut the fibrotic hepatic parenchyma. The higher pressure leads to more vessel injuries without coagulation function, especially of the hepatic veins, which corresponds to a higher blood loss. Although the new water jet is added with electoral cautery for hemostasis, it does not work simultaneously.
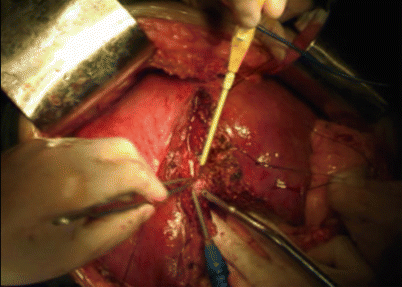

Fig. 8.2
Transection of the liver parenchyma with water jet dissector to fragment the liver parenchyma tissue and leave the vascular and ductal structures for subsequent treatment
8.4.4 Radiofrequency (RF)-Assisted Devices
In the 2000s, the RF-assisted device has been used for hepatic parenchyma transection by creating thermal coagulative necrosis along the transection plane, followed by transection of the coagulated liver using a simple scalpel [27, 28]. This method used an RF needle originally designed for ablation of liver tumors, rather than for liver transection [29], and has been reported to be a useful technique for hepatic resection. This early RF-assisted device is monopolar probe. However, this technique was found to be time consuming, produced uncontrolled amount of energy and excessive amount of dead tissue, and also carried the risk of skin burns from the grounding pad [30].
To address these problems, Habib’s group designed and developed a bipolar RF device, the Habib 4X [31]. The probes are introduced into the liver along the transection plane. The generator is programmed to produce thermal coagulation. This allows a small, less than 10 mm, margin of coagulated liver parenchyma to remain behind ensuring sealed vessels and bile ducts. The probes are introduced again adjacent to the last coagulated area, in a serial fashion, until the area to be transected is ablated. The surgeon can either apply energy to the whole resection margin and then cut or apply energy to a partial section and then cut that section and repeat it (Fig. 8.3). RF-assisted liver resection has been shown to be effective in reducing intraoperative blood loss [31–34]. Moreover, RF-assisted liver resection potentially increases the margin of clearance, thus providing an oncological advantage [35]. However, this technique has the limitation of potential damage to the major intrahepatic bile duct or vessels because there is a risk of the needle being inserted into or near a major intrahepatic segmental bile duct or vessel. Thus its application close to the hilum and the inferior vena cava requires experience and dissection of the hepatic hilum and the hepatic veins before applying this device close to these structures. Left lateral lobectomy needs exposure of biliary duct before application of this device. Besides, the amount of tissue necrosis in the remnant liver is substantial, especially when the transection area is large. This is a major concern when patients with cirrhosis and limited liver function reserve require major hepatic resection [35]. In addition, some researchers reported that RF-assisted liver resection caused a higher rate of both bile leak and abdominal abscess formation and needed a longer operation time compared with clamp crush [36].
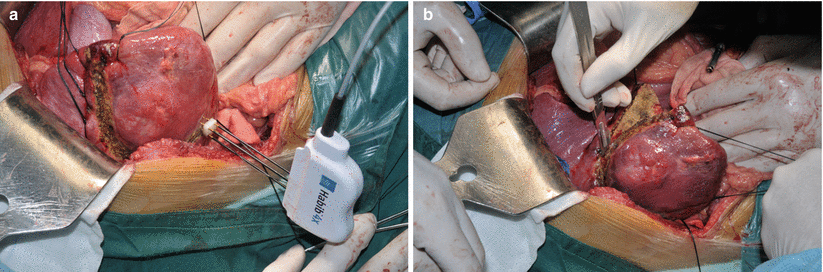

Fig. 8.3
Liver resection with bipolar radiofrequency device: Habib 4X. The probe is introduced into the transection line to cause the coagulative necrosis (a), and then cut that section (b)
8.4.5 Harmonic Scalpel
Harmonic scalpel, firstly introduced in the 1990s, is an ultrasonic surgical device that simultaneously cuts and coagulates. During liver transection, this technology uses ultrasonically activated shears to seal small vessels between the vibrating blades (Fig. 8.4). The blade’s longitudinal vibration can dissect liver parenchyma easily, creating heat and thereby denaturing protein to form coagulum. Vessels up to 2–3 mm in diameter are coagulated on contact with the vibrating blade. The tissue-cutting effect derives from a saw mechanism in the direction of the vibrating blade [37]. Additionally, the temperature of the harmonic scalpel is less than 80 °C when dissecting, far lower than that of the electrome (150 °C) and thus far less damage to the surrounding tissues. Precise dissection around the important tissues could be performed by harmonic scalpel.
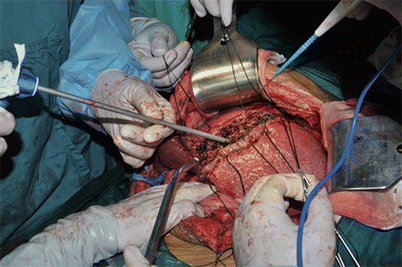

Fig. 8.4
Use of harmonic scalpel in open liver resection, the liver parenchyma is coagulated and divided by the coagulating shears
But it is reported that harmonic scalpel was associated with a significantly increased rate of postoperative bile leakage, raising the concern that harmonic scalpel may not be effective in sealing bile ducts [38]. This remains to be proven by a randomized trial, which is not available in the literature yet. The instrument may also be limited to dissect the liver parenchyma around the main trunk of hepatic veins, since it is difficult to achieve sufficient control of bleeding from large vessels using the harmonic scalpel alone [39].
Although the benefit of the use of harmonic scalpel in open liver resection remains uncertain, harmonic scalpel with the longer arm is commonly used in laparoscopic liver resection and could achieve excellent results, especially for resection of peripheral lesions. The harmonic scalpel may also be useful in transection of cirrhotic liver, for which the clamp crush and water jet may not be very effective [37].
8.4.6 The LigaSure Vessel Sealing System
The LigaSure vessel sealing system (LVSS) was introduced into clinical practice for liver resection in the 2000s and developed for transection and hemostasis, rather than a standard hemostasis technique [40, 41]. Its wide use in hemorrhoidectomies, neck operations, and pulmonary resections has been well reported [42–44]. The device uses compression pressure and powerful bipolar radiofrequency energy; it causes shrinkage of collagen and elastin between opposing walls of small- and medium-sized blood vessels as well as bile vessels. It can seal effectively and create a permanent seal of arteries up to 7 mm and veins up to 12 mm in a wide variety of clinical applications [45]. It is more efficient than ultrasonic shears for hepatic resection in a porcine model [46].
The LVSS can be used alone for liver transection or in combination with clamp crushing to seal vessels [47]. The use of LVSS improves surgical results via reducing blood loss and transfusion and postoperative complications such as bile leakage and intra-abdominal abscess [45, 48–50]. Similar to the harmonic scalpel, LVSS is a useful instrument for liver transection in the setting of laparoscopic resection of peripheral liver lesions. In one study, LVSS is shown to be effective for liver transection in normal or near-normal liver but to fail to achieve hemostasis in three patients with cirrhotic liver [51]. Nevertheless, the usefulness of LVSS has been highlighted.
8.4.7 Vascular Stapling Devices
Vascular stapling devices have been suggested as alternative instruments for parenchymal transection [52, 53]. Stapler hepatectomy in patients with a diseased liver may, moreover, be supported by the ability to perform resections without routine use of inflow occlusion. The technique is simple and easy to learn and master. Advantages of the stapler technique include a fast transection with potentially reduced intraoperative hemorrhage and postoperative bile leakage due to a highly standardized closure of vascular and biliary structures. Another point that must be taken into account is that dividing the portal branches and the hepatic vein using the stapler is already a central part of many hepatic procedures. The use of endoscopic vascular staplers is a feasible, safe, attractive approach for dividing liver parenchyma during routine hepatic surgery. The results are comparable to those obtained using the CUSA without additional cost. However, the use of a stapler for transection of the liver parenchyma may be applicable in minor wedge resection or left lateral segmentectomy when the liver tissue is not too bulky [37].
8.5 Comparison of Different Liver Transection Techniques
Liver resection comes along with risks, and the rate of complications remains high. Therefore, the need to reduce such complications has led to the development of various innovative methods of liver resection. During the past decade there has been a significant increase in the number of liver resections and various new device applications [54]. However, sufficient evidence has still not been accumulated to establish the most effective method, and liver surgeons still select the method of liver transection according to their own preferences.
8.5.1 Modern Instructions Versus Clamp Crush in Liver Transection
Clamp crush has been generally considered to be the standard method for liver parenchymal transection over the past decades [55]. Even in the well-equipped center, the liver surgeons should have this basic ability. Of four meta-analyses comparing the technology-assisted versus clamp-crush liver resection, the results are shown in Table 8.1. The latest outcomes by Alexiou including all RCTs or non-RCTs may be relatively best evidenced. According to Table 8.1, the current evidence-based medicine demonstrates that: of the alternative methods used in liver resection (LVSS, CUSA, hydrojet, harmonic scalpel, and RFDS), only LVSS appeared to offer significant benefit over standard clamp crush regarding blood loss, postoperative bile leak, and hospital stay. Clamp crush is quicker than CUSA, hydrojet, and RFDS and cheaper than the other methods. RFDS could reduce the blood loss but is associated with a higher rate of intra-abdominal abscess than the clamp-crush method. Nevertheless, further well-designed trials are required to warranty the usefulness of LVSS and RFDS. LVSS has been successfully used in many surgical subspecialties [43] but has only recently been introduced in liver surgery, and the experience of most surgeons is rather limited. Thus, they may be reluctant to change their standard practice.
Table 8.1
Meta-analysis comparing the modern instructions versus clamp crush in liver transection
Study | Comparison with clamp crush | Results |
|---|---|---|
Gurusamy et al. (2009) [56] | CUSA (2 RCTs), RFDS (2 RCTs), hydrojet (1 RCT), sharp dissection (1 RCT) | Infective complications and transection blood loss were greater in the RFDS than in clamp crush Clamp crush is quicker than CUSA, hydrojet, and RFDS and cheaper than the other methods No significant differences in the mortality, morbidity, or hospital stay in the other comparisons |
Rahbari et al. (2009) [55] | CUSA (3 RCTs), LVSS (1 RCT), hydrojet (1 RCT), sharp dissection (1 RCT) | No difference between alternative transection method and clamp crush in terms of blood loss, transection time, morbidity, biliary leakage, and hospital stay |
Alexiou et al. (2013) [57] | LVSS (3 RCTs and 3 non-RCTs), CUSA (4 RCTs and 1 non-RCT), RFDS (3 RCTs and 3 non-RCTs) | LVSS has lower blood loss, lower risk for bile leak, and shorter hospital stay and similar parenchyma transection time and mortality compared with clamp crush No difference was observed between CUSA or RFDS and clamp crush for any of the abovementioned outcomes |
Xiao et al. (2014) [58] | RFDS (4 RCTs and 5 non-RCTs) | Total intraoperative blood loss and blood loss during liver transection were lower in RFDS RFDS is associated with a higher rate of intra-abdominal abscess than the clamp-crush method No significant difference was observed between both the groups for the incidence of both blood transfusion and bile leak |
The stapler technique is the relative novel modality used in liver resection. Thus far, there is only one RCT for the stapler technique versus clamp crush [19]. A total of 130 patients were enrolled in this study; there was no difference between groups in total intraoperative blood loss. But blood loss during parenchymal transection was significantly lower in the stapler transection group that is due to the shorter of parenchymal transection time in the stapler group. There were no significant differences in postoperative morbidity or mortality between groups.
8.5.2 Comparison Among Modern Instructions
There have been several studies to date, including randomized controlled trials, comparing the clinical benefits of different methods of liver transection. However, few if any data have been presented to suggest that one transection technique has advantages over another [22, 59–62]. Presently, it can be concluded that no specific tool and/or approach has been found superior to the other when it comes to the liver parenchyma transection. This was also the conclusion of a recent systematic review of the literature [55, 56]. In fact, even today the standard of method in hepatic surgery is to divide the tissue by use of simple devices such as Kelly clamp technique. Despite these conclusions, reached from an evidence-based platform, it is clear that many expert centers across the world also prefer to use the CUSA to divide the parenchyma. A generally advocated opinion is that the CUSA allows the hepatic surgeon to complete and master more difficult and meticulous dissections, particularly along the hepatic pedicles and major vessels.
The choice of transection techniques is currently a matter of preference of surgeons, as there are few obvious evidences that suggest one transection technique has advantages over another. Probably the best option should be a combined approach, making full use of each advantage.
8.6 Complications and Treatment
With the arrival of the precise hepatectomy age, the overall complication rate has often been reported to be markedly decreased, but hemorrhage and bile leakage remain major complications after liver parenchyma transection. Hemorrhage is one of the most serious complications, and re-laparotomy is frequently required to control active hemorrhage. Bile leakage and biloma formation present major obstacles for an uneventful recovery after liver resection [63].
8.6.1 Hemorrhage
The incidence of hemorrhage usually occurs within 48 h with about 10 % [64, 65]. The incidence of life-threatening hemorrhage requiring re-laparotomy varies from 1 to 8 % [66, 67]. Three common reasons for hemorrhage are: (1) bleeding from the transection surfaces, which may be a consequence of arterial branch truncation or congestion of the hepatic vein due to stenosis or ligation; (2) incomplete intraoperative hemostasis, such as inappropriate manipulation of the hepatic vein root or trauma to the diaphragm, and increased vena cava pressure; and (3) vascular sutures loosened or fallen off, an event which usually is ascribed to elevated pressure in the vena cava from patients’ body movement, such as turning over or coughing severely [68]. Thorough intraoperative hemostasis is critical and must be ascertained before the operation is concluded. When the root of the hepatic vein is suspected to be injured intraoperatively, hemorrhage from the vein or the inferior vena cava should be carefully sought by increasing the intrathoracic pressure artificially. Mattress sutures with hepatic needles should be used for the hemostasis, and the traumatized surface can be covered with gelatin sponge, biological glue, or omentum as means of achieving additional hemostasis. Close monitoring of vital signs and transfusion of whole blood, platelets, and plasma are usually recommended to ensure the patient’s blood pressure and pulse remain stable. Otherwise, re-laparotomy should be considered.
Indications for re-laparotomy after hepatectomy were hemorrhage resulting in serious hypovolemic shock intractable to fluid resuscitation and/or blood transfusion, such as persistent low blood pressure (systolic pressure < 60 mmHg or pulse pressure < 20 mmHg), more than 200 ml of blood from an abdominal drain in 10 min, and a drop of >3 g/dl of hemoglobin within 1 h despite ongoing blood transfusion. At re-laparotomy, the abdominal cavity was explored to look for any active bleeding site, which was then managed by ligation, suturing, or repair of the vessel wall. Pringle maneuver should be avoided to prevent further ischemia–reperfusion injury to the liver, unless the bleeding from the surface was so massive that the surgical field was obscured. When no definite bleeding site was identified and only extensive oozing was observed, the raw wounds were treated with argon beam coagulation. If oozing continued, gauze compression packing was used for hemostasis. In summary, careful manipulation during operation and thorough hemostasis and drainage are critical for success in attaining hemostasis.
8.6.2 Bile Leakage
The incidence of bile leakage ranges from 6 % to 17 % [69–71]. Common causes of postoperative bile leakage are: truncation of the distal bile duct in the residual liver, the most common cause, and injury of the bile duct from inappropriate surgical technique. Traumatized liver surface area, intraoperative blood loss, and operative time were reported as the independent risk factors for bile leakage after hepatectomy [72, 73]. During surgery, the residual liver can be covered with wet gauze, in which the presence of minimal bile seepage may predict postoperative bile leakage. To avoid postoperative bile leakage, biological glue can be applied to the surface of the residual liver [74]. Postoperative monitoring should include observing for abdominal pain, rebound tenderness, muscle tension, and bile leakage from the drainage tube. In addition, CT or MRCP can be used to determine if the bile duct is occluded and, if so, where the occlusion is located. The bile leakage may resolve spontaneously within two months. However, if peritonitis develops, open surgery should be performed as soon as possible for thorough cleaning of the abdominal cavity and repair of the damaged bile duct. In general, nonoperative treatment was sufficient if the results of MRCP and CT were negative for bile leakage, but operative intervention was needed if conservative therapy failed. Moreover, we recommend the reoperation of patients who present significant bile leakage during the first 24 h after the operation.
8.6.3 Liver Failure
Liver failure is a severe postoperative complication of hepatectomy, even liver transplantation is required to save lives. It is closely associated with active hepatitis, cirrhosis, small residual liver volume, the duration of hepatic portal vein occlusion, and even the perioperative medication used. An incidence of liver failure after hepatectomy of about 8 %-10 % has been reported [75, 76]. Some common preventive measures are more important: carefully assess the liver’s functional reserve and reduce intraoperative bleeding, especially for patients with liver cirrhosis. After hepatectomy, the patient should be closely monitored, with particular attention to abnormalities in levels of consciousness, liver function, the volume and character of drainage fluid, and serum lactic acid levels. Acidosis is very common in liver failure, so the level of serum lactic acid should be carefully monitored. Serum bilirubin level should rapidly decrease. If the level increases abruptly after the second postoperative day, the risk of hepatic failure increases. Comprehensive therapy for liver failure includes postoperative supplementation with albumin, fibrinogen, or prothrombin complex and transfusion of fresh blood.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







