Type
Location of PVTT
Vp0
No PVTT
Vp1
PVTT in portal branches distal to the second branches
Vp2
PVTT in the second portal branches
Vp3
PVTT in the first portal branches
Vp4
PVTT in the main portal trunk or contralateral portal branch
16.3.2 PVTT Classification of the Eastern Hepatobiliary Hospital
Cheng et al. [14] analyzed the clinical data of Eastern Hepatobiliary Hospital and put forward a new PVTT classification system based on the part of the portal vein invaded by the PVTT. In this system, PVTTs are divided into types I0–IV, of which types I–III are divided into two subtypes each (Table 16.2, Fig. 16.1). Research reveals that types I–III have satisfactory surgical outcome, especially types I and II, while types III and IV present poor prognoses. Additional investigation suggests that this classification system is more accurate with respect to stratification and prognosis prediction than the TNM, CLIP, and JIS systems [25].
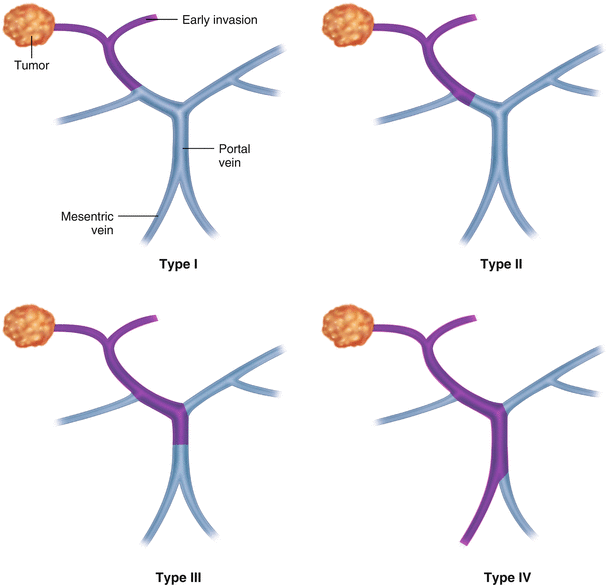
Table 16.2
PVTT classification of the Eastern Hepatobiliary Hospital
Type | Subtype |
|---|---|
Type I0: PVTT in histological examination | |
Type I: PVTT invades the second or smaller branches of the portal vein | Type Ia: PVTT invades the third or smaller branches of the portal vein |
Type Ib: PVTT invades the second portal branches | |
Type II: PVTT invades the first portal branch | Type IIa: PVTT invades the first portal branch of one lobe (the left or right portal trunk) |
Type IIb: PVTT invades the first portal branch of two lobes (the left or right portal trunk) | |
Type III: PVTT invades the main portal trunk | Type IIIa: the PVTT is no more than 2 cm beneath the bifurcation of the portal vein |
Type IIIb: the PVTT extends more than 2 cm beneath the bifurcation of the portal vein | |
Type IV: PVTT invades the superior mesenteric vein |

Fig. 16.1
PVTT classification of the Eastern Hepatobiliary Hospital
16.3.3 Other Classification of PVTT
Some scholars divide PVTT into types A–C, based on the tumor’s location and surgical outcome [15] (Fig. 16.3). Hepatectomy is suggested for type A. Hepatectomy and thrombectomy via the stumps are performed in type B. In type C, the wall of the portal trunk should be incised to extract the PVTT. The mean postoperative survival time is 9 months in type A and B, while it is only 5 months in type C (Table 16.3).
Table 16.3
Other PVTT classifications
Type | Location of the PVTT |
|---|---|
Type A | Within the resection area |
Type B | Extends to the first portal branches, exceeding the resection line by 1–2 cm |
Type C | Extends to the main portal trunk or the contralateral portal branches |
16.4 Preoperative Evaluation
16.4.1 Radiological Evaluation
1.
Due to its high sensitivity and specificity, ultrasound is the preferred radiological examination for PVTT. Due to its advantages, such as it being simple, intuitive, noninvasive, radiation-free, and inexpensive, it is also the most widely used examination method. Ultrasound can clearly demonstrate the cavity and blood flow of the portal vein. Ultrasound can also detect abnormal echoes and distinguish them from the thrombus caused by cirrhosis based on its blood supply and properties. Pulsatile blood flow detected by color Doppler flow imaging makes the diagnosis of PVTT easier (Figs. 16.2, 16.3, 16.4, and 16.5).
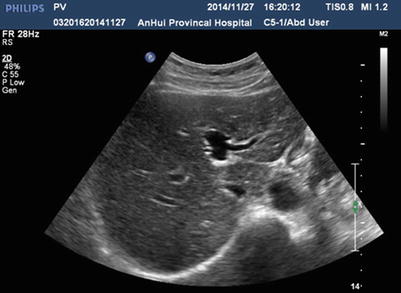
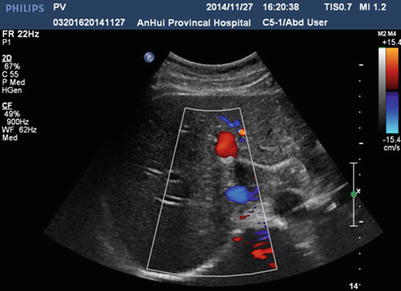
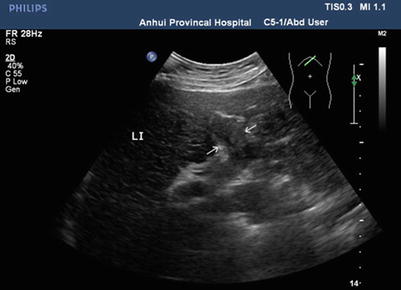
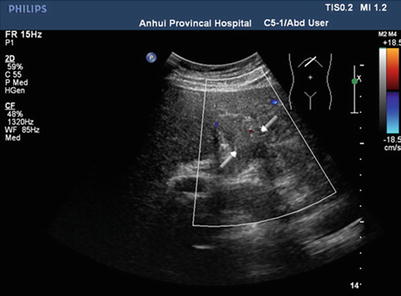

Fig. 16.2
Normal sonography of the portal vein. The left portal branch is unobstructed with no PVTT in the cavity

Fig. 16.3
Normal sonography of the portal vein. Colorful blood signal is present in the cavity of the left portal vein, indicating patency

Fig. 16.4
Gray-scale ultrasonography of PVTT, PVTT in the left portal branch (arrows)

Fig. 16.5
Color Doppler ultrasonography of PVTT, color Doppler ultrasonography detects no blood signal in the left portal branch due to PVTT occlusion. There is little blood in the periphery of the related portal vein (arrows)
2.
Computed tomography (CT) is one of the most important radiological examinations for the diagnosis and differential diagnosis of liver tumor. CT can reveal the morphology and blood supply of the liver tumor and is conducive to detection, identification, staging, and posttreatment follow-up. In the diagnosis of PVTT, CT can provide information of the tumor’s size, location, and relationship with adjacent large vessels. In addition, CT can help identify the mass lesion in the portal vein and differentiate PVTT from thrombus (Figs. 16.6 and 16.7). CT, especially dynamic enhanced spiral CT, has relatively high resolution, which facilitates the precise localization of PVTT. The CT indications of portal vein thrombus are a thickened cavity and a low-density filling defect in the cavity. Indirect indications are enhanced portal vein wall, the formation of collateral circulation, and cavernous transformation of the portal vein.
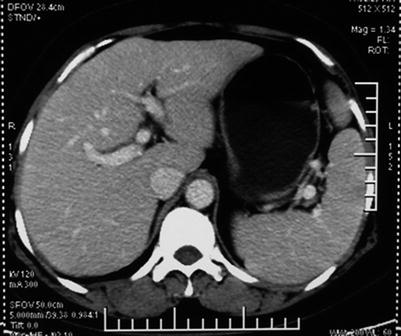
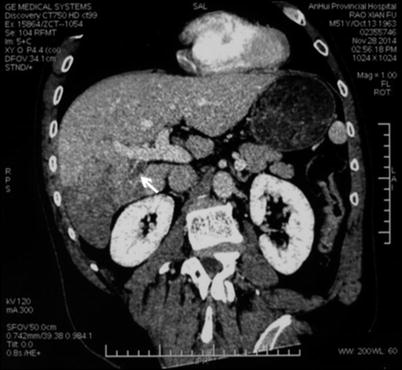

Fig. 16.6
Normal CT image of the portal vein. The cavities of the left and right portal branch are clear, with no filling defect

Fig. 16.7
CT image of PVTT. The tumor is located in the right liver, with a PVTT in the right posterior branch of the portal vein. The arrow marks the filling defect in the cavity of the right posterior branch of the portal vein
3.
Magnetic resonance imaging (MRI) has the same value as CT in PVTT diagnosis. MRI can provide cross-sectional, coronal, and sagittal images and evaluate the size and location of the PVTT. It can also evaluate the extent of portal vein occlusion. On MRI, PVTT exhibits normal signal intensity or hypointensity on T1WI image and hyperintensity on T2WI image, with no significant post-enhanced changes. This pattern is similar to the pattern of the liver tumor. MRI has high sensitivity and specificity for PVTT (Figs. 16.8 and 16.9).
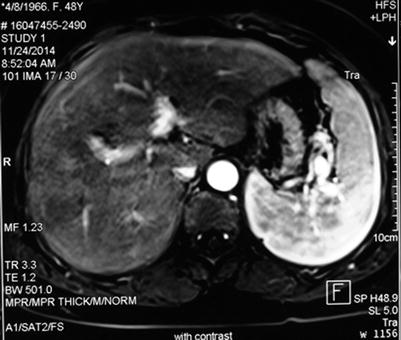
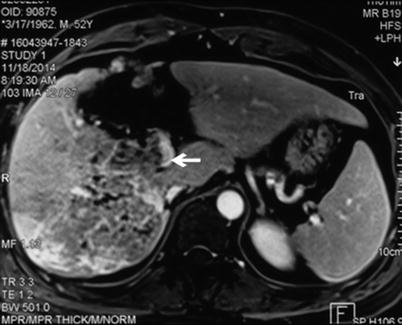

Fig. 16.8
Normal MRI image of a portal vein. The cavities of the left and right portal branch are clear, with no abnormal signal intensity

Fig. 16.9
Image of a PVTT. The tumor is located in the right liver, with a PVTT in the right posterior branch of the portal vein. The arrow marks hypointensity on enhanced MRI in the cavity of the right posterior branch of the portal vein. The hypointensity extends to the bifurcation of the portal vein
4.
The liver surgery planning system has some advantage in the diagnosis and treatment of liver tumors associated with PVTT. The liver surgery planning system can calculate the liver remnant and simulate the liver surgery based on: (1) the preoperative overall assessment of hepatic functional reserve, (2) images of the tumor and liver anatomy, and (3) the local condition of the tumor and its relationship with adjacent important vessels [26]. 3-D image reconstruction reveals a defect in the portal vein when it is occluded by a PVTT. If the PVTT is significant, PVTT reconstruction can be performed (Fig. 16.10). A CT image is shown in Fig. 16.11.
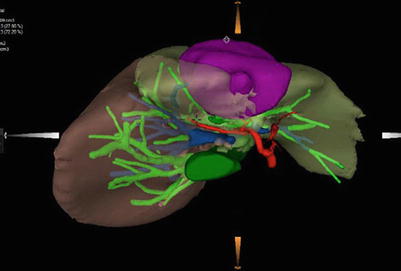
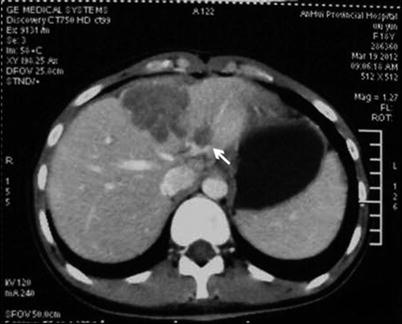

Fig. 16.10
3-D image reconstruction of PVTT. The tumor is located in the left liver, with a PVTT in the left portal branch. The arrow marks the total occlusion of the left portal branch by the PVTT

Fig. 16.11
The tumor is located in the left medial lobe, with a PVTT in the left branch of the portal vein (arrows)
5.
PET-CT combines PET with CT. PET reveals the biochemical and metabolic condition of the hepatic mass lesion and can precisely locate the lesion. PET-CT provides cross-sectional images of the whole body in one examination and has advantages with respect to sensitivity, specificity, and precision. Thus, PET-CT can diagnose and detect metastasis in the early stage. In theory, PET-CT can differentiate PVTT from thrombus. However, in clinical practice, the diagnostic accuracy of PVTT is unsatisfactory, and this technique now serves only as a supplemental examination.
16.4.2 Assessment of Hepatic Functional Reserve
Post-hepatectomy liver failure is an important cause of perioperative mortality and is the main predictor of short-term survival of patients with hepatocellular carcinoma associated with PVTT. A precise assessment of the hepatic functional reserve is very important for selecting the treatment protocol, determining the resection scope, and reducing the risk of post-hepatectomy liver failure.
Many methods are now available for evaluating the hepatic functional reserve. Apart from plasma biochemical tests and scoring systems (e.g., the Child-Pugh score and the MELD score), the indocyanine green (ICG) excretive test can objectively demonstrate the hepatic functional reserve and provide an important reference value with respect to the selection of the method and timing of surgical treatment [27]. Generally speaking, a Child-Pugh A patient with an ICGR15 of <10 % can tolerate a large-volume liver resection of four segments. A Child-Pugh A patient with an ICGR15 of 10–19 % can tolerate a large-volume liver resection of 2–3 segments. A Child-Pugh A patient with an ICGR15 of 20–29 % can tolerate liver resection of only one segment. When the ICGR15 is 30–39 %, the patient can only tolerate a small regional liver resection. When the ICGR15 is ≥ 40 %, only enucleation is permitted [28].
The ICG excretion rate varies greatly with the hepatic blood flow. Therefore, the factors that influence hepatic blood flow, such as PVTT, post-portal vein embolization, and regional blood flow disturbance, all influence the test results. The location of the PVTT and the extent of the occlusion should be considered preoperatively [29].
Calculating the volume of the remnant liver by CT or MRI is a simple but effective way to assess the hepatic functional reserve and can help predict and prevent post-hepatectomy liver failure.
Zurich University combined portal hypertension, the Child-Pugh score, the future liver remnant, the ICGR15 value, and the condition of liver parenchyma (i.e., with or without cirrhosis) and put forward a relatively objective and reasonable method for assessing hepatic functional reserve [30].
In 2011, Chinese experts reached a consensus with respect to the preoperative assessment of hepatic functional reserve. In this consensus, ICGR15, the Child-Pugh score, radiological evaluation of liver parenchyma and vessels, and the calculation of liver volume are combined to form an evaluation system to determine the safe limit of liver resection. This consensus is very important for the individualization of the method and the scope of liver resection [31].
16.4.3 Preoperative Evaluation
The patient’s overall condition, the preoperative hepatic functional reserve, the location of the tumor, and the condition of the PVTT are all factors that influence the surgery and the prognosis.
The general condition of patients can be evaluated using the ECOG scoring system. Other aspects that need assessment are nutrition; water-electrolyte balance; acid-base balance; and the conditions of vital organs, such as the heart, lungs, and kidneys.
Preoperative evaluation of hepatic functional reserve is conducive to assessing the patients’ tolerance level of hepatectomy and thrombectomy, thus providing a basis for surgical planning and reducing post-hepatectomy liver failure.
Radiology not only plays an important role in preoperative hepatic functional reserve evaluation but also determines the PVTT classification. Ultrasound, CT, and MRI can show the lesions of the liver parenchyma, assess the hepatic functional reserve, and indicate the safety of liver surgery. In addition, these methods can classify the PVTT, help with surgical planning, and predict the prognosis. CT can also provide a 3-D reconstruction, allowing a simulated hepatectomy via the liver surgery planning system.
16.5 Surgical Indications
According to the BCLC strategy for staging and treatment, early-stage hepatocellular carcinoma (stage 0-A) is indicated for radical resection. PVTT belongs to stage C, which requires targeted therapy with sorafenib instead of surgery [20]. The updated Japan Society of Hepatology (JSH) 2010 guidelines for the management of hepatocellular carcinoma proposed PVTT in the portal branches as an indication for hepatectomy [32]. In 2014, the NCCN Clinical Practice Guidelines in Oncology proposed that large-vessel invasion is indicated for hepatectomy, despite debate [33]. In 2014, Hong Kong University proposed the Hong Kong Liver Cancer staging system with treatment stratification. The proposed indications for hepatectomy are a single tumor, diameter ≤ 5 cm, intrahepatic vessel invasion, and Child-Pugh A [21].
There is no consensus regarding the surgical indications for hepatocellular carcinoma accompanied by PVTT. Generally accepted indications are: (1) the general condition is fine, with no organic disease of vital organs; (2) liver function is normal or only slightly damaged (Child-Pugh A), or Child-Pugh B improves to Child-Pugh A after short-term treatment; and (3) the liver tumor is localized without metastasis. Based on the PVTT classification proposed by LCSGJ, Vp1, Vp2, and Vp3 are indicated for resection, while Vp4 is a relative indication. Based on the PVTT classification proposed by Cheng et al. in Eastern Hepatobiliary Hospital, types I, II, and III are indicated for resection, while type IV is a contraindication.
16.6 Preoperative Preparation
1.
Medical history and physical examination: In the patient’s medical history, low back pain caused by liver tumor metastasis should be given special attention. Upon physical examination, lung metastasis, ascites, and cachexia should be given special attention. Several tests should be run, such as routine blood work, blood biochemistry, blood coagulation, serum AFP, abdominal ultrasound, CT, MRI, and PET-CT, when necessary.
2.
Evaluation of hepatic functional reserve: Child-Pugh scoring combined with an ICG excretive test is most widely used. Vitamin K and prothrombin complex concentrate are administered to those with coagulation abnormities. Albumin is administered to those with hypoproteinemia. For those with damaged liver function, treatment aimed at improving liver function should be continued until it is essentially normal. Vitamins, glucose, and albumin should be given to undernourished patients. Anemia should be treated.
3.
Evaluation of the function of the vital organs, such as the heart, brain, and kidney: Seek professional help when necessary to reduce surgical risk.
4.
Administration of laxatives to clean the gut the day before surgery, if necessary.
5.
Preparation of blood for intraoperative transfusion according to the scope of resection.
16.7 Operative Method Options
Among all of the treatments for PVTT, surgery has the highest probability of cure. Most PVTT growth occurs from the original tumor to the portal trunk, which is the foundation for radical resection. This procedure removes the tumor and the PVTT in one operation. There are three widely used operative methods, which are described below.
16.7.1 Hepatectomy
Hepatectomy is the most radical operation and consists of resecting the tumor and PVTT en bloc. This procedure can be performed if the PVTT does not extend beyond the first branch of the portal vein and the patient’s general condition and liver function are suitable for left or right hemihepatectomy. For tumors in the right anterior lobe, a right anterior lobectomy can be performed in which the PVTT is resected together with the right anterior portal branch if the PVTT does not exceed beyond the right anterior portal branch. Similarly, for tumors in the right posterior lobe, if the PVTT does not exceed beyond the right posterior portal branch, a right posterior lobectomy can be performed in which the PVTT is resected together with the right posterior portal branch.
16.7.2 Hepatectomy + PVTT Extraction through the Liver Cross Section
This procedure applies to PVTT extending to the portal bifurcation or trunk, exceeding the resection line by 1–2 cm. After resecting the liver, occlude the portal trunk and open the portal stumps on the liver cross section to extract the PVTT by clamping, flushing, or suction. If blood exits from the stump during reperfusion, the portal vein is most likely clear. Intraoperative ultrasound can detect whether the PVTT is totally extracted. Before extraction, the contralateral portal branch is temporarily occluded to prevent cancer cell metastasis to the contralateral portal vein.
16.7.3 Hepatectomy + Thrombectomy through the Portal Trunk
If the PVTT extends to the portal trunk or the contralateral portal branch, the portal trunk needs to be incised to extract it. Expose the portal trunk and its left and right branches, occlude the proximal end of the portal trunk, and incise it from its bifurcation. If the PVTT adheres loosely to the vessel wall, it can be extracted gently. If the adhesion is tight, en bloc resection can be considered. The resected portal vein can be mended using an autogenous vein, vascular prostheses, or autogenous peritoneum. End-to-end anastomosis of the portal vein is another option [5].
16.8 Detailed Surgical Procedures
16.8.1 Hepatectomy
Consider a case of left hemihepatectomy as an example. This case is an 18-year-old female patient with a history of HBV infection since childhood. The serum AFP level is 17,654 ng/ml, the Child-Pugh score is A, ICGR15 is 3.9 %, and the diagnosis is hepatocellular carcinoma associated with PVTT. The tumor is located in the left medial lobe, with a PVTT in the left branch of the portal vein not exceeding the first portal branch. Anatomical left hemihepatectomy is performed, and the left portal branch is resected en bloc with the PVTT. A 3-D reconstruction image is shown in Fig. 16.10, and a preoperative CT image is shown in Fig. 16.11.
Get Clinical Tree app for offline access

1.
The patient is placed in a supine position. Measures are taken to keep the patient warm, and elastic stockings are put on to prevent deep venous thrombosis. Make an oblique incision beneath the right costal margin; resect to the xiphoid to directly expose the inferior vena cava. After exploring the abdominal cavity, intraoperative ultrasound is routinely performed to detect the number and size of the lesion, its relationship with surrounding vessels, and other small lesions beyond the planned resection area (Figs. 16.12 and 16.13).

Fig. 16.12
An oblique incision is made beneath the right costal margin