Martin K. Kuhlmann, Peter Kotanko, Nathan W. Levin
Hemodialysis
Outcomes and Adequacy
From an idealistic clinical perspective, an adequately treated hemodialysis (HD) patient is physically active, well nourished, euvolemic, and normotensive with a maintained good quality of life and a life expectancy that is not inferior to that of healthy patients. Despite these optimistic goals, outcomes for dialysis patients expressed in terms of mortality, hospitalization, and quality of life regrettably remain comparable to those observed in patients with solid organ cancer. The mean life expectancy of an average 60-year-old HD patient currently can be estimated at 5 to 7 years, with an annual mortality rate of 13% to 20% (www.usrds.org). Mortality in HD patients differs by gender and race; for example, in the United States there is lower mortality in African Americans compared with Caucasians, in Hispanics compared with non-Hispanics, and in Asians compared with any other racial group. Data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) suggest that variations in practice patterns, such as prescribed dialysis treatment time, average erythropoietin dose, preferred vascular access type, and number of patients with central venous catheters, may account for some of these differences. Outcomes are also directly related to comorbid factors occurring during evolution of chronic kidney disease (CKD), such as atherosclerotic vascular disease, diabetes, and arterial hypertension, and to background cardiovascular mortality of the respective general population.1 Indeed, the majority of CKD patients die mainly of cardiovascular causes before reaching end-stage renal disease (ESRD). In addition to cardiovascular disease (discussed further in Chapter 82), other major factors influencing outcome that are common to all patients with ESRD include anemia (see Chapter 83) and control of bone and mineral metabolism (see Chapter 85).
In this chapter, we discuss dialysis-related factors influencing outcome in patients receiving maintenance HD and measures to influence these factors clinically (see summary in Table 94-1). A key factor influencing outcome is “adequacy” of dialysis, a term which was originally used exclusively to describe dialysis dosage measured by small solute removal, but now has a broader meaning encompassing all aspects of the replacement of excretory and endocrine functions of the kidney that affect outcome.
Table 94-1
Factors related to dialysis outcome and ways to address these factors.
BMI, Body mass index; CKD-MBD, chronic kidney disease–mineral and bone disorder; CPAP, continuous positive airway pressure; EPO, erythropoietin; FGF-23, fibroblast growth factor 23; Hb, hemoglobin; HbA1c, hemoglobin A1c; HD, hemodialysis; HDF, hemodiafiltration; Kt/V, dialysis treatment index; PTH, parathyroid hormone; UFR, ultrafiltration rate; URR, urea reduction ratio.
| Factors Related to Dialysis Outcome | |
| Factors | Ways to Address These Factors |
| Patient Characteristics | |
| Age | None |
| Gender | None |
| Race/ethnicity | None |
| Body size | Target BMI above 23 kg/m2; treat malnutrition if BMI is falling |
| Social status | Social and family support |
| Dialysis vintage | Early renal transplantation |
| Comorbidities | |
| Diabetes mellitus | Prevent hypoglycemia, target HbA1c below 7.5% |
| Arterial hypertension | Optimize target weight, decrease dietary salt intake, avoid intradialytic positive sodium balance |
| Congestive heart failure | Optimize fluid status by gradual postdialysis target reduction, decrease dietary salt intake, avoid intradialytic positive sodium balance |
| Depression | Treat medically |
| Peripheral vascular disease | Aspirin, lipid-lowering substances, management of hyperphosphatemia |
| Transplant eligibility | Living donor transplantation |
| Frailty | Nephrologic rehabilitation program, exercise during dialysis to avoid falls and fractures |
| Physical activity | Nephrologic rehabilitation program, exercise during dialysis; avoid immobilization |
| Arrhythmias | Avoid hypokalemia and prevent hyperkalemia; do not use zero-K+ dialysate bath |
| Obstructive sleep apnea | Optimize fluid status; use CPAP if indicated |
| Anemia | |
| Hemoglobin concentration | Target Hb level 10-11 g/dl |
| EPO resistance | Surveillance of iron status; target transferrin saturation >25%; identify and treat sources of inflammation |
| CKD-MBD | |
| PTH | Lower phosphate levels toward normal range, use vitamin D and calcimimetics |
| FGF-23 | Lower phosphate levels toward normal range |
| Hyperphosphatemia | Optimize dialysis phosphate elimination; phosphate binder therapy, dietary phosphate restriction |
| Nutrition and Inflammation | |
| Inflammation | Ultrapure dialysate, avoid central venous catheters and graft access |
| Low serum albumin | Ultrapure dialysate, avoid central venous catheters and graft access |
| Protein-energy malnutrition | Nutritional counseling, enteral supplements |
| Fluid overload | Define post-HD target weight, bioimpedance? |
| Vascular access | Avoid central venous catheters and graft access |
| β2-Microglobulin | Increase middle molecule clearance, HDF |
| Dialysis Treatment Factors | |
| Session length | Session length more important than urea clearance once minimum Kt/V has been achieved; minimum session length 4 h? |
| Frequency | Four to seven sessions per week; short daily in-center HD, daily or every-other-day nocturnal home HD |
| Long interdialytic interval | Every-other-day dialysis or four sessions per week |
| Dialysis dose | Target Kt/V >1.3; URR >65% per treatment |
| Dialysis modality | High efficiency on-line HDF; target convective volume >22 l per treatment |
| Hemodialysis membrane biocompatibility | Biocompatible membranes |
| Hemodialysis membrane type | High-flux membranes |
| Dialysate quality | Ultrapure dialysate |
| Dialysate composition (acidosis, hyperkalemia) | Individualized dialysate bicarbonate, potassium, calcium, and sodium concentration |
| Acidosis | Target pre-HD HCO3− >22 mmol/l, avoid severe post-HD alkalosis |
| Hyperkalemia | Nutritional counseling, oral bicarbonate supplementation |
| Timing of dialysis initiation | Avoid early dialysis initiation without clinical symptoms of uremia |
| Intradialytic hemodynamic stability | Thermoneutral dialysis, avoid high UFR |
| Residual renal function | Avoid intradialytic hypotension, volume depletion, and high UFR |
Adequacy of Dialysis Dose
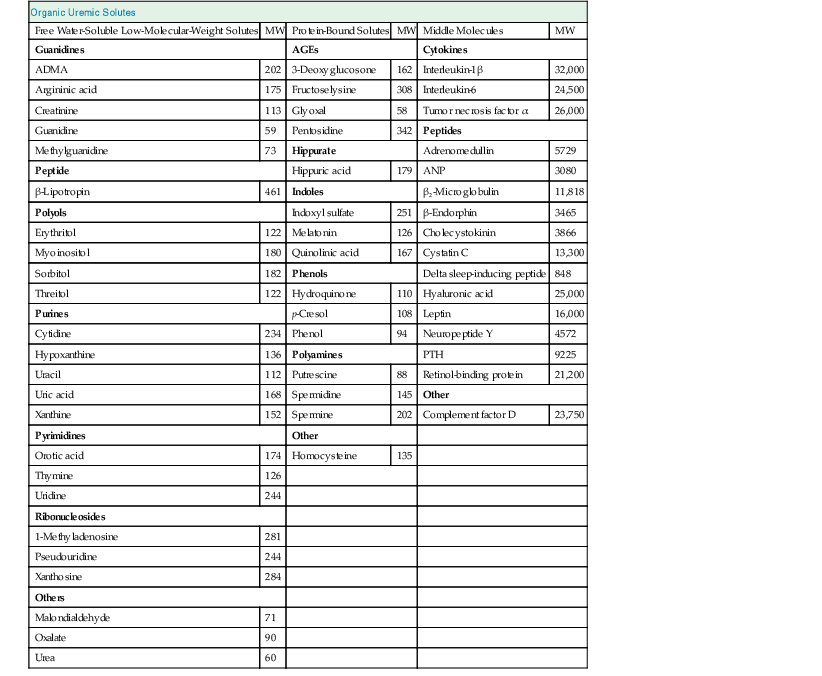
Uremic Toxins
The retention in the body of compounds that normally are metabolized by healthy kidneys or filtered and secreted into the urine results in the uremic syndrome. These retained compounds are called uremic retention solutes or uremic toxins when they interact negatively with biologic functions. Uremic toxins include a small group of inorganic compounds, such as water, potassium, phosphate, and trace elements, and a much larger group of organic compounds that are further subdivided into small water-soluble solutes (<500 d), middle molecules (>500 d), and protein-bound solutes (Table 94-2). A continuously updated list of uremic toxins is provided at www.uremic-toxins.org. The catalogue of uremic toxins is long, and a growing number of compounds have been associated with outcomes. Mortality has repeatedly been shown to be associated with reduced clearance of urea. Of commonly measured middle molecular size substances, serum concentrations of β2-microglobulin have been associated with mortality, as have concentrations of p-cresyl sulfate among protein-bound uremic solutes.2 Although it is evident that uremic toxicity is more than the retention of urea or water-soluble compounds alone, current dialysis and dialysis-related treatments do not remove any significant quantity of substances larger than 10 to 15 kd. Future means of removing higher-molecular-weight toxins or protein-bound substances may include the use of sorbents in addition to traditional diffusive and convective dialysis strategies.
Urea as a Surrogate Marker of Uremic Toxicity
Among all potential uremic toxins, only urea, a 60-dalton small water-soluble compound, is established as a marker of uremic solute retention and removal. Urea, which itself shows little toxicity, is a metabolite of amino acid metabolism; urea generation therefore depends on protein intake and the balance between protein anabolism and catabolism. The removal of urea was originally considered to be representative for the removal of other water-soluble solutes with a higher pathogenic impact, but it is now clear that urea removal does not closely parallel that of other small water-soluble compounds, protein-bound solutes, or middle molecules. This is because urea, in contrast to other uremic solutes, is freely diffusible through cell membranes, which allows rapid equilibration of urea concentration within whole body water after urea has been removed from the blood compartment. For other uremic toxins the intercompartmental transfer rate is much slower, leading to a protracted equilibration between the intravascular and other tissue compartments. Nevertheless, knowledge of urea kinetics is essential for the general understanding of the physics of solute removal during dialysis.3
Assessment of Dialysis Dose
Adequacy in terms of dialysis dose refers to delivery of a treatment dose that is considered sufficient to promote optimal long-term outcome. The delivered dose of dialysis is conventionally assessed by the removal of urea and expressed either by the urea reduction ratio (URR) or by the treatment index Kt/V (see later for detailed explanation). Simply following predialysis blood urea nitrogen (BUN) or serum urea concentration is misleading; low serum urea may much more reflect malnutrition from inadequate protein intake than adequate dialysis urea removal. If urea removal is inadequate, then dialysis is inadequate, regardless of the serum urea level.
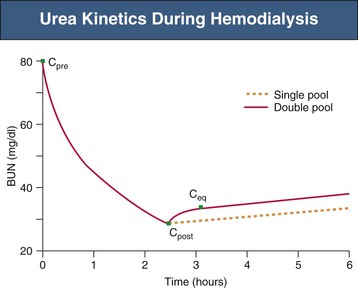
Intradialytic Urea Kinetics
Dialyzers are highly efficient at removal of urea from the blood, reducing the urea concentration during one passage by 80% to 90%. Intradialytic serum urea kinetics were originally described by single-compartment (single-pool) models, assuming that full equilibration between blood and tissue compartments occurs immediately and without time delay. In such models, the change in serum urea follows first-order kinetics, with a linear decline and no urea rebound (Fig. 94-1, dashed line). In vivo, however, intercompartmental urea redistribution is somewhat delayed, and therefore the intradialytic urea concentration in the blood compartment is always lower than in tissue compartments. Full equilibration among all compartments is reached within 30 to 60 minutes after the end of a dialysis treatment. These features of urea kinetics are more accurately described by double-pool models that take into account the intercompartmental urea redistribution rate and postdialysis urea rebound (Fig. 94-1, solid line).
Current methods for assessment of dialysis dose are based on the predialysis and postdialysis difference in serum urea and include URR, single-pool Kt/V (spKt/V), equilibrated Kt/V (eKt/V), and weekly standard Kt/V (std-Kt/V).
Urea Reduction Ratio
Urea reduction ratio refers to the treatment-related reduction of serum urea concentration and is computed as follows:
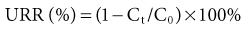
where Ct is postdialysis and C0 is predialysis serum urea concentration.
Urea reduction ratio is a simple but rather imprecise way to quantify dialysis dose because it does not take into account intradialytic urea generation and convective urea removal by ultrafiltration. Despite these limitations, URR correlates well with dialysis outcome and is an accepted method for assessment of dialysis adequacy. A minimum URR of 65% to 70% is recommended for adequate HD.
Single-Pool Kt/V (spKt/V)
The treatment index Kt/V is the most widely used parameter to assess dialysis dose. Kt/V is a dimensionless number representing the total plasma volume cleared (K × t, in liters) divided by volume of distribution (V, in liters). The concept of Kt/V may be applied to any substance but in clinical practice is used almost exclusively for urea, where K is the dialyzer blood water urea clearance (liters per hour), t
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree