Peter Kotanko, Martin K. Kuhlmann, Nathan W. Levin
Hemodialysis
Principles and Techniques
Despite the widespread use of peritoneal dialysis and renal transplantation, hemodialysis (HD) remains the main renal replacement therapy in most countries. Currently more than 2 million patients are treated with HD in about 28,500 dialysis units worldwide. Despite substantial advances in our understanding of the biology of chronic kidney disease (CKD) and the risk factors for poor outcome on HD and improvements in dialysis technology, the annual mortality in HD patients varies from 5% to 25% internationally, depending on demographic and possibly genetic factors.
Dialysis System
The aim of the HD system is to deliver blood in a fail-safe manner from the patient to the dialyzer, enable the efficient removal of uremic toxins and excess fluid, and deliver the cleared blood back to the patient. The main components of the dialysis system are the extracorporeal blood circuit, the dialyzer, the dialysis machine, and the water purification system.1 The dialysis machine delivers dialysis fluid with the intended flow rate, temperature, and chemical composition. It has monitoring and safety systems for air, blood, conductivity, and pressure; blood and dialysate pumps; a heating system; a dialysate mixing and degassing unit; and a volumetric ultrafiltrate balancing system. The role of the water purification system is to produce water for dialysis that complies with international chemical and microbiologic standards.
Dialyzer Designs
The dialyzer provides controllable transfer of solutes and water across a semipermeable membrane. The flows of dialysate and blood are separated and countercurrent. The dialyzer has four ports, one inlet and one outlet port each for blood and dialysate. The semipermeable dialysis membrane separates the blood compartment from the dialysate compartment. The transport processes across the membrane are diffusion (dialysis) and convection (ultrafiltration). The removal of small solutes occurs primarily by diffusion, whereas larger components such as β2-microglobulin are more effectively removed by convection. The hollow fiber dialyzer is currently the most effective design; it delivers high dialysis efficiency with low resistance to flow in a small device.
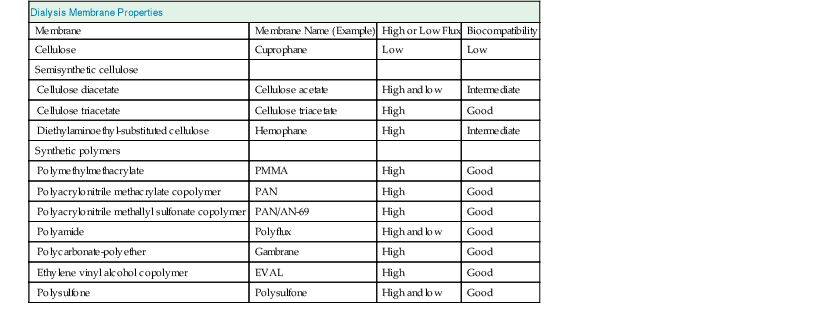
Dialysis Membranes
Membranes vary with respect to chemical structure, biophysical properties such as transport characteristics, and biocompatibility (Table 93-1).
Materials
The original widely used membrane material was cellulose, which is made up of repetitive polysaccharide units containing hydroxyl groups. In cellulose acetate 80% of the hydroxyl groups are replaced by acetate radicals. Synthetic tertiary amino compounds are added during cellulose membrane synthesis to form cellulosynthetic membranes. More recent membranes are not cellulose based but instead are built of entirely synthetic materials, such as polyacrylonitrile, polysulfone, polycarbonate, polyamide, and polymethylmethacrylate. These synthetic membranes provide superior biocompatibility and are widely used.
Transport Properties
Transport of molecules across the dialysis membrane occurs because of (1) the concentration gradient (diffusive transport) and (2) the hydrostatic pressure gradient across the membrane (convective transport) and is dependent on membrane pore size. Dialyzer efficiency in terms of urea removal depends on the surface area (usually 0.8 to 2.1 m2). High-efficiency dialyzers have a high surface area irrespective of pore size and possess a superior clearance for small molecules but may have small pores and thus a low ability to remove large molecules such as β2-microglobulin. The dialyzer mass transfer area coefficient (KoA) for urea is a measure of the theoretically maximal possible urea clearance (in milliliters per minute) at infinite blood and dialysate flow rates. Dialyzer efficiency can be categorized according to KoA for urea as low (<500 ml/min), moderate (500 to 700 ml/min), and high (>700 ml/min). In contrast to the dialyzer clearance of a substance, its KoA is independent of flow rates in the blood and dialysate compartments. High-flux dialyzers have pores large enough to allow the passage of larger molecules such as β2-microglobulin (molecular weight 11800 d). Water permeability is defined by the ultrafiltration coefficient (Kuf) in milliliters of transmembrane ultrafiltration per hour per millimeter of mercury of transmembrane pressure (ml/h/mm Hg). Kuf is high in high-flux dialyzers, with Kuf up to 80 ml/h/mm Hg. During high-flux HD, backfiltration (the flow of dialysate into the blood because of higher hydrostatic pressure on the dialysate side) can occur at the distal end of the dialyzer blood compartment and may result in a transfer of 5 to 10 liters of dialysate into the blood during a treatment; it is compensated for by an increased blood water removal at the proximal part of the dialyzer. Therefore, water quality is of paramount importance when high-flux dialyzers are used.
In the prospective, randomized Membrane Permeability Outcome (MPO) study a survival benefit of high-flux membranes was seen among patients with serum albumin levels of 4 g/dl or lower, and in diabetic patients.2 These results are in contrast with the results of the Hemodialysis (HEMO) study, which did not show any effect in patients with hypoalbuminemia, but did show survival benefit in patients on dialysis for longer than 3.7 years before the trial.3 These differences may be related in part to population characteristics (race, recruitment of incident of prevalent patients), or to the fluxes achieved.
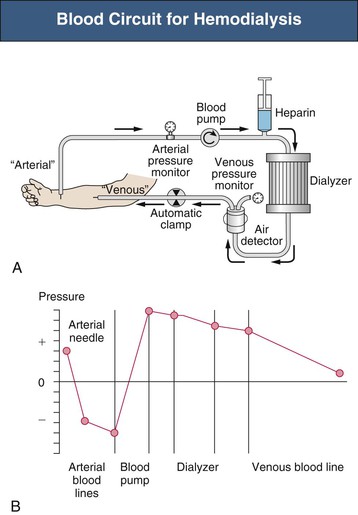
Safety Monitors
Safety monitors are integral parts of the dialysis machine. Pressure monitors are integrated in most machines to monitor the system pressure in critical positions (Fig. 93-1)1:
A venous air detector and air trap are located downstream of the venous pressure monitor. A positive signal at the air detector automatically clamps the venous line and stops the blood pump. A blood leak detector is placed in the dialysate outflow line. Dialysate temperature is constantly monitored. Dialysate is produced by a proportioning system that mixes acid and bicarbonate concentrates with purified water. The osmolarity of the dialysate translates into conductivity, which is measured by the dialysis conductivity monitor. The ultrafiltration rate must be controlled precisely, in most machines by a volumetric control system.
Anticoagulation
Either unfractionated heparin or, in some countries, low-molecular-weight heparin (LMWH) is used to prevent blood clotting in the extracorporeal circuit. Constant infusion of heparin, repeated boluses of heparin, or a single bolus of LMWH may be used. LMWH has not been approved by the U.S. Food and Drug Administration (FDA) for use in dialysis patients in the United States. A number of alternative modalities are available for patients at high risk for bleeding or who have contraindications to heparin, such as saline flushes, regional citrate anticoagulation (RCA), prostacyclin, danaparoid, argatroban (direct thrombin inhibitor), and lepirudin (recombinant hirudin). Lepirudin has the disadvantage of an extremely prolonged half-life in dialysis patients. In some institutions, RCA is used routinely, especially in patients with recent surgery, coagulopathies, thrombocytopenia, active bleeding, pericarditis, or heparin-associated side effects (such as heparin-induced thrombocytopenia type 2, pruritus, rapidly progressive osteoporosis, and alopecia). Neutrophil activation may be reduced with RCA compared with heparin anticoagulation. Heparin requirements may be reduced with the use of citrate-containing dialysate.4
A typical routine prescription of constant infusion heparin is to administer an initial bolus of 2000 IU followed by a heparin infusion (800 to 1200 IU/h) ending 30 to 60 minutes before the end of the session. Applying the repeated-bolus method, an initial heparin bolus of, for example, 4000 IU is followed by, for example, 1000 to 2000 IU after 2 hours. Although the half-life of LMWH may be prolonged in renal failure, it has proved safe with fewer bleeding episodes, if appropriate dose reductions are made. LMWH has become the anticoagulant of choice in many centers for routine HD. LMWH is given as a bolus at the beginning of the session. Routine monitoring of whole-blood partial thromboplastin time, activated clotting time, or factor Xa is usually not necessary.
Dialysate Fluid
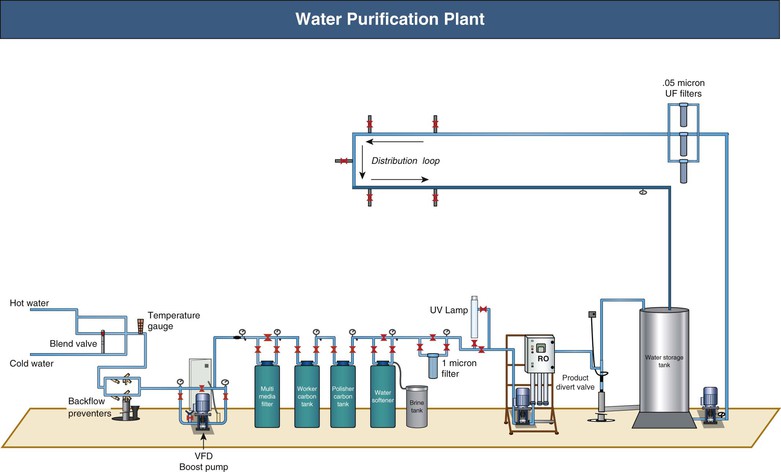
Water and Water Treatment
A standard 4-hour HD session exposes the patient to 120 to 160 liters of water. Therefore, water quality is of paramount importance to the patient’s well-being. A typical water purification plant is shown in Figure 93-2. Water from municipal sources is filtered to remove particulate matter. Activated carbon, with high surface area, adsorbs substances such as endotoxins, chlorine, and chloramines. Downstream water softeners use a resin coated with sodium ions, which are exchanged for calcium and magnesium ions, before the water enters the reverse osmosis (RO) system. During RO, water is pumped at high pressure (15 to 20 bar) through a tight membrane. The small pore size of these membranes (0.5 to 0.5 nm) provides an absolute barrier for molecules larger than 100 to 300 d. This process rejects over 99% of all bacteria, viruses, pyrogens, and organic materials. Thereafter water is pumped into the RO storage tank and from there into a loop that supplies the dialysis stations. Common faults include carbon filters with inadequate capacity and recharging with inadequate frequency. Optional ultraviolet irradiation, to be placed upstream of a filter, is used to damage bacteria.