18
Growth Hormone Replacement in Aging Men
Case History
Mr. J.M. is a 50-year-old white man who was referred by a colleague because of his interest in optimal aging and antiaging issues. The patient has been around the world seeking management of his health issue. In reality, he is healthy and is just keen on preserving his functionality and maintaining his health. He realizes that there is no real “fountain of youth,” but he expressed interest in growth hormone (GH) replacement. In fact, he is seeking a second opinion, as his previous physician had already started him on growth hormone after testing him and determining he had low insulin-like growth factor-I (IGF-I) levels. He is currently on 16 IU of growth hormone injections per week. On direct questioning, he admitted to feeling tired and slow prior to the therapy. He also said that the therapy has helped maintain his body fat at ∼15%, which was previously in the 20% range. He exercises daily with weights for ∼20 minutes and does aerobic exercises for another 20 minutes. He does not smoke, but he drinks socially. He loves life and travels the world for ballooning adventures. He is also a successful businessman and is semiretired. His only significant past history is gastroesophageal reflux, and he finds relief with Nexium. Examination reveals a healthy person, weighing 170 pounds and with a height of 5 feet 10 inches. Skin turgor was normal and within the range for his age. Body fat by caliper was 13%. Right hand grip strength was 110 pounds, with a grip differential of 10 pounds. Short-term visual memory was above average for his age. Neurological and cardiovascular examinations were within normal limits. Blood pressure was normal and he did not have any evidence of carpal tunnel syndrome. There was also no swelling noted.
Introduction
Many of the physical changes associated with aging mimic those seen in the adult GH-deficiency syndrome, such as muscle atrophy, osteopenia, obesity, cardiovascular deterioration, exercise intolerance, decreased metabolic rate, dyslipidemia, thinning of the skin, and low quality of life in terms of energy and social life.14 The successful treatment of adult GH deficiency with recombinant human GH has raised the possibility that GH supplementation could reverse or slow down some of the deleterious features of aging. This chapter discusses GH, specifically its biosynthesis, secretion, effects, relation to aging, use, the secretagogues, and the future uses of this hormone.
Structure and Biosynthesis of Growth Hormone
Human GH is a 191-amino-acid single-chain polypeptide with a molecular weight of 22 kd. GH is made as a prehormone by somatotrophs, which are acidophilic cells in the pars tuberalis of the anterior lobe of the pituitary gland.5 GH circulates in the plasma bound to a specific GH binding protein (GHBP)—GHBPI.6 The circulating half-life of GH is ∼20 to 25 minutes.7
Regulation of Synthesis and Secretion of Growth Hormone
Growth hormone secretion is controlled by two factors secreted in the hypothalamus. Hypothalamic growth hormone–releasing hormone (GHRH) is the major stimulator of GH synthesis and secretion. GHRH is a 44-amino-acid peptide. The major inhibitor of GH synthesis and secretion is somatostatin (SS), another hypothalamic 14-amino-acid peptide. Both are secreted into the capillaries of the median eminence and then pass via the portal veins to the pituitary gland.5 The interplay of stimulation and inhibition is further modulated by a local negative feedback by GH itself on the pituitary. Fig. 18–1 summarizes the regulation and secretion of growth hormone.

FIGURE 18-1. Summary of regulation and secretion of growth hormone (GH). GH secretion has both direct and indirect effects. The direct effects are the result of GH binding its receptor on target cells. Adipocytes have GH receptors and GH stimulate them to break down triglyceride to free fatty acids. The indirect effects are mediated by insulin-like growth factor-I (IGF-I), which is secreted by the liver in response to GH. The majority of growth promoting effects of GH is due to IGF-I, a chip on target cells.
The secretion of GH is characteristically pulsatile. In humans there are at least five to seven pulses lasting 1 to 2 hours.8 About two thirds of daily GH secretion occurs at night, with pulses occurring especially during slow-wave sleep. This pulsatility is in turn modulated by pulsatile GHRH stimulation.9 There are other factors that can stimulate GH synthesis and secretion, and these include hypoglycemia; α-adrenergic, dopaminergic, and serotonergic agents; and amino acids such as arginine.7 Besides SS, hyperglycemia, free fatty acids, and β-adrenergic agonists can inhibit synthesis and secretion of GH.7
Physical exercise is a physiological stimulus to GH secretion. Repeated bouts of aerobic exercise in a 24-hour period has been shown to result in increased 24-hour integrated GH concentrations. However, the effect of chronic resistance training on 24-hour GH release is still not well established.10 Exercise not only stimulates GH secretion but also causes a direct increase in circulating IGF-I independent of GH.11,12
GH secretion is also under the control of a stimulatory pathway that may be activated by some synthetic compounds, the GH secretagogues (GHSs). Examples of these secretagogues are the GH-releasing peptides MK-0677 and hexarelin. These secretagogues bind to the GHS receptor (GHS-R), a family of G-protein–coupled receptors found in the hypothalamus and the pituitary. They act via the phosphatidyl inositol triphosphate pathway to stimulate and amplify GH release.13
Much interest has surrounded a 28-amino-acid peptide, Ghrelin, which is a natural ligand for the GHS-R first identified by Kojima and colleagues.14 Rat studies have revealed that it is produced mainly in the stomach but also in the duodenum, ileum, cecum, aorta, thyroid, lung, and testes. Ghrelin and GHS exert many similar effects by stimulating and augmenting the release of GH, and they also stimulate the release of prolactin, adrenocorticotropic hormone (ACTH), and cortisol. Ghrelin is involved in energy homeostasis. In humans, Ghrelin was shown to stimulate food intake and also reduces cardiac afterload and increases cardiac output.13 Figure 18–2 summarizes the regulation of Ghrelin.
The Actions of Growth Hormone and Insulin-Like Growth Factors
Besides being essential for normal growth in childhood, GH has many direct and indirect effects on metabolism, body composition, and immunity in adults. The direct metabolic effects of GH include increased protein synthesis, increased mobilization of fatty acids from adipose tissue, and decreased rate of glucose utilization throughout the body, resulting in increased blood sugar.5,15 The indirect effects are through the stimulation of the production of insulin-like growth factors IGF-I and IGF-II. Of the two, IGF-I is the more important.
GH stimulates IGF-I production in the liver16 and in several other tissues.17 The major source of circulating IGF-I comes from the liver. In other tissues, IGF-I acts on other cells in a paracrine manner.18 Circulating IGF-I levels depend mainly on GH. IGFs, including IGF-I, are bound to circulating proteins, six in all, known as IGF binding proteins (IGFBPs). IGFBP-3 is the most abundant and accounts for 95% of the total circulating IGFBPs in adults. It binds 80 to 90% of IGF-I and increases the half-life of IGF-I by 100-fold.19 Compared with the half-life of GH, that of IGF-I is much longer, 12 to 15 hours.17
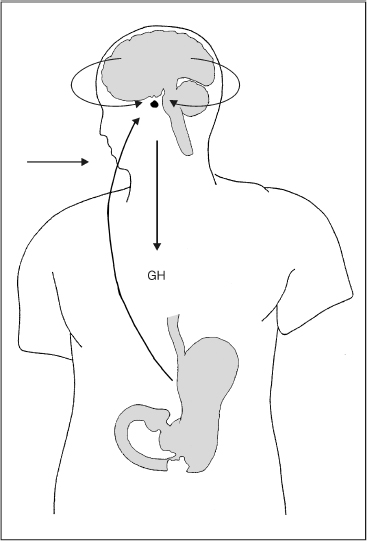
FIGURE 18-2. Regulation of Ghrelin, a natural ligand for GHS-R that exerts effects similar to GHS by stimulating and augmentating the release of GH via “the pituitary-stomach axis.” GH is produced and secreted from the anterior pituitary gland and exerts its effects by binding to GH receptor. Ghrelin on the other hand, is found in the stomach and has been discovered to regulate GH, suggesting the presence of a “pituitary-stomach” axis. (Adapted from Kojima M, Hosoda H, Matsuo H, et al. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab 2001;12:118–126, with permission.)
Growth Hormone and Insulin-Like Growth Factor-I in Aging
GH secretion is reduced in older adults, arising from a gradual decline of GH with aging.20 The rate of decline is ∼14% per decade of adult life.21 The decline probably starts in the third decade and approaches a plateau from about age 60 years.22,23 It must also be noted that GH levels in age-related decline of GH are higher than in adult GH deficiency secondary to disease.24,25 Levels of IGF-I also fall with age.26 The age-related reduction of IGF-I is also observed as early as the third decade.27 The decline in older age takes the IGF-I levels to ∼50% of the levels in healthy young adults.15 Table 18–1 summarizes the symptoms and signs of growth hormone deficiency.
| Symptoms | Signs and Physiological Effects |
| Psychological problems | ⇓ Lean body mass |
| Poor general health | ⇓ Extracellular volume |
| Impaired self-control | ⇓ Bone mineral density |
| Lack of positive well-being | Increased body fat |
| Mood changes, including depression, anxiety | Increased waist-hip ratio |
| Reduced vitality | ⇓ HDL, ⇑ LDL |
| Sexual dysfunction | ⇓ GFR ⇑ Renal plasma flow |
| Tiredness | ⇓ BMR |
| Emotional problems | ⇓ Muscle bulk and strength |
| ⇓ Exercise performance | |
| ⇓ Anaerobic threshold |
BMR, basal metabolism rate; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The age-related decline of GH is due to a decrease in the stimulation of pituitary GH secretion by the hypothalamic GHRH and the increased inhibition of pituitary GH secretion by SS.25 The pulse amplitude in particular declines with age.4,22 The pituitary somatotrophs appear to retain adequate or even unchanged secretory capacity in older adults.
It has been questioned whether the increase in body fat and decrease in lean body mass is a consequence of age-dependent decline of GH or is the accumulation of fat mass secondary to factors such as diet and lifestyle, which has led to a feedback inhibition of GH release.28,29
The Effect of Growth Hormone Administration in Age-Related Decline of Growth Hormone
There is no controversy about the indications for the use of GH for childhood growth failure, adult growth hormone deficiency (e.g., secondary to pituitary irradiation), and AIDS-associated wasting.30 But GH replacement in the elderly for age-related decline still remains controversial. Rudman et al’s31 study in 1990 on the effect of GH replacement in the elderly is now considered a landmark. They recruited 21 healthy men between the ages of 61 to 81 years of age. These men had IGF-I levels below 350U/L during a 6-month baseline period. They received 0.03 mg of biosynthetic human growth hormone per kilogram weight subcutaneously three times a week. A control group of nine men received no treatment. At the end of 6 months, the mean plasma IGF-I level rose to 500 to 1500U/L in the treatment group. The levels remained at less than 350U/L in the control group. The members of the treatment group had increased lean body mass and skin thickness, decreased adipose tissue mass, and an increased average lumbar vertebral bone density.
Following this there were other studies.32,33 All have shown that treatment with GH increased lean body mass, decreased adipose tissue mass, and increases skin thickness. Groups elsewhere have found evidence of improvement in body composition but no functional improvement. Papadakis et al,34 who studied the effect of GH or placebo administered to 52 healthy elderly men with low IGF-I levels over a period of 6 months, also reported no improvement in functional status in those who had received GH. Recently a double-blind placebo controlled study by Blackman et al35 on 27 women and 25 men reported similar findings.
Goh and colleagues36 studied 23 healthy elderly Chinese men between the ages 60 and 69. They were given subcutaneous injections of 0.08 U/kg recombinant GH for 6 months. Three months after starting the study, it was found that these men showed a significant increase in lean body mass and a decrease in body fat. He also found that there were GH-induced increases in thyroid function and serum dehydroepiandrosterone sulfate (DHEAS) levels. The effects of thyroid hormone on metabolism are well known. It has been suggested that DHEAS may have an antiobesity effect, and may enhance memory by improving rapid eye movement (REM) sleep, increase muscle mass, activate immune function, and enhance quality of life in aging men.
The effect of GH on bone mass is less clear. In a review, Geusens and Boonen37 found that most studies have shown that administration of GH in healthy elderly individuals resulted in stimulation of bone formation and resorption. In a placebo-controlled study, 12 months of GH administration had a beneficial effect on bone density, preventing the age-related reduction of bone mass seen in the placebo group but not increasing bone density in absolute terms.38 Studies have shown that GH administration did not affect bone density but did prevent the reduction of bone mass.39,40
Problems in the Study of Age-Related Decline of Growth Hormone
Although observations on metabolic changes were repeatable in all studies, study design of the effect of GH therapy for age-related decline in otherwise healthy individuals remains a problem. More robust evidence would be obtained if large, randomized, double-blind studies were done. However, one can foresee problems in the conduct of such studies. To begin with, what should be the entry criteria to such a study? Is it going to be based on clinical parameters or on measurements of GH and/or IGF-I? Also, it is known that asymptomatic patients may have low levels of IGF-I, whereas symptomatic patients may not have low levels of IGF-I.17 So initially there must be studies establishing the relationship between signs of aging on the one hand and the decline of GH and IGF-I on the other. This will not be easy because aging is a complex process involving physiological, psychological, social, and endocrine functions. Another question is how and when shall GH be measured, knowing its secretion is pulsatile, that the majority of its secretion is at night, and that the half-life of GH is short.
Current methods to assess the integrity of GH secretion are mainly the GH stimulation tests, which utilize the factors that trigger the release of GH, namely insulin, arginine, and GHRH. The insulin tolerance test (ITT) induces hypoglycemia and challenges the body’s ability to respond by producing GH. However, 10% of normal individuals fail to respond to hypoglycemia, so other tests may be necessary. There are contraindications to such tests, especially in diseases where hypoglycemia is a risk factor, for example, epilepsy. In addition, the test itself is not without risk. Hypoglycemia may be so significant and severe as to cause ketosis acidosis and shock.41 To further complicate matters, a study on the use of GH in aging should include patients 65 years of age and over (i.e., the elderly) and the American Academy of Clinical Endocrinologists does not recommend the ITT for patients older than 65 years of age.30
GH-releasing peptide is also used to stimulate the production of GH by the pituitary. Arginine infusion and GHRH injection have been combined, and the results are apparently comparable with those of the ITT.7 Arginine alone has also been used to challenge pituitary GH production, and its infusion has been known to raise GH by 70% in healthy individuals.7
Considering the problems and complexity associated with challenge tests of GH production, for clinical purposes of management it should be enough to assess serum IGF-I as an indicator of GH levels. Serum levels are fairly stable and do not exhibit a diurnal rhythm; they reflect a composite of daily GH secretion rates. Therefore, a single IGF-I level is a reasonable though indirect measure of the 24-hour GH secretion.16 However, it has been argued that IGF-I production decreases in the aging liver and that this fact would confound the level measured.42 Furthermore, IGF-I levels are influenced by many other variables. Malnutrition, severe chronic illness, severe liver disease, and hypothyroidism reduce plasma IGF-I levels.
Studies have taken a predetermined lower percentile of the IGF-I range for young adults as reflective of decline in GH production in the elderly.31,36 Bearing in mind the limitations of IGF-I as a biological parameter, thought could be given to establishing a clinical score, much in the same way as is done for osteoporosis, where an assessed low value of serum IGF-I is evaluated as the number of standard deviations below the mean serum IGF-I of healthy young adults. Such a score, although likely to be indicative of GH secretion, will have to be associated with defined clinical end points before it can be widely applicable.
Therefore, for any two studies to be comparable, the methods used to assess the GH-IGF-I axis and the criteria used to define age-related decline must be the same. Another problem in any trial on GH supplementation and aging would be the definition of end points. These shall have to be grouped into physical and psychological end points if studies were to have a holistic impact.
For physical end points, results can be objective; a percentage change in a physical parameter is unequivocal. But how much of a change shall be deemed an appropriate response? Psychological end points are harder to define. Papadakis and colleagues34 used the Mini-Mental State Examination to evaluate improvement in cognitive function when they looked at a 6-month replacement of GH in 52 healthy elderly men. Although standardized psychological tests are useful, they are to some extent affected by the subject’s level of education, attitudes, religious beliefs, and life experiences before and during the period of the test. The psychological and social status of the subject may have changed over the time of the test such as death in the family or onset of morbidity, such as development of cataracts and strokes.
Moving on to the subjects themselves, one must be realistic and accept that, given the demographics of the study population, a high proportion of individuals will be excluded from the study over time. Morbidity and mortality is increased from illnesses to which aging adults are prone, such as strokes, hypertension, diabetes, and cancer. Life situations for the subjects may change, precluding them from suitability during the study, which may affect the assessment of psychological end points. These include the death of a spouse or loss of financial support and change in home situations, for example, moving from home to an institution. In earlier studies, the high incidence of side effects in the use of GH led to a high rate of dropouts.32,38
The Approach to the Patient
Given the widespread publicity surrounding GH, the clinician is not uncommonly faced with the dilemma of the approach to an otherwise healthy patient who presents with signs and symptoms attributable to the age-related decline of GH. The dilemma arises for the following reasons and questions:
1. There is a lack of robust evidence or a guide from well-designed clinical trials with meaningful end points.
2. The laboratory diagnosis is problematic and may be controversial.
3. There is a known higher incidence of prostate and breast cancer in subjects with high IGF-I.8 There are reports suggesting that acromegalic patients have an increased risk of colon cancer, colonic polyps, and malignancies of the lymphoid system.1,43 Rosen et al,1 however, pointed out that the production rate of GH in a GH producing adenoma far exceeds the usual recombinant GH (rGH) substitution dose.
4. Is an age-related decline of GH a normal protective mechanism, assuming that administration of GH would result in the survival of aging cells, which have a higher tendency to abnormally proliferate, perhaps leading to cancer?
5. How long should treatment be given?
6. The cost of treatment is high. GH replacement in patients with GH deficiency secondary to disease could cost between $7,000 and $10,000 annually.44
7. There are side effects from GH administration, although these are usually dose related. Side effects include edema, arthralgia, myalgia, carpal tunnel syndrome, glucose intolerance, and hypertension.33,43
8. The exclusion of its use in patients with coexistent illness such as diabetes mellitus.
9. The possibility of development of tolerance by patients to administered rGH. In Thompson et al’s32 study although nitrogen retention remains significantly higher than baseline values throughout the study it had declined by the final week of treatment.
A reasonable approach to a patient who is potentially suffering from symptoms and signs associated with age-related decline of GH is one of exclusion of all other causes, for example, hypothyroidism, depression, anemia, and others. The clinician should start with a thorough and complete history including a review of systems. This should then be followed by a meticulous clinical examination. Laboratory tests should include a full blood count, tests of liver and renal functions, serum lipids, cancer markers, and a full endocrine evaluation.
It is not suggested that GH should be offered to the patient as a panacea for obvious concerns addressed earlier. Because signs and symptoms associated with GH decline are not life-threatening, it is only appropriate to consider conservative measures. These are a healthy lifestyle including proper nutrition, appropriate exercise, avoidance of smoking, avoidance of drug and alcohol abuse, and social interactions to maintain good mental health and promote a healthy environment.
The salutary effect of exercise on GH production has been alluded to earlier. Taaffe and colleagues45 performed a double-blind, placebo-controlled exercise trial involving 18 healthy elderly men who underwent progressive weight training for 14 weeks. Subjects then either received 0.02 mg/kg body weight daily GH subcutaneously or received a placebo while undergoing a further 10 weeks of strength training. They found that although lean body mass increased and fat mass decreased, there was no significant difference in muscle strength observed between the two groups. Thus exercise alone could achieve the increase of lean body mass and decrease of fat mass seen with GH administration. Hurel et al46 studied GH production in 10 male subjects running over 40 miles per week as compared with 10 healthy age-matched sedentary males. They found that GH was higher in the runners.
Given the cost, complexity, and potential risks of GH use, attention is now turning to GH-releasing peptides, nonpeptidyl secretagogues, and GHRH.43 As mentioned earlier, it has been shown that the pituitary gland retains its capacity to secrete GH even in the aging population. Thus it is logical to administer agents, ideally by the oral route, to induce release of GH by the pituitary. The benefit is that GH itself has negative feedback on the pituitary, and the risk of excess GH levels in the individual is thus avoided. In other words the release of GH by secretagogues is autoregulated.
There are biosynthetic peptides that could be administered nasally and orally that act on receptors in the hypothalamus and pituitary gland to stimulate the release of GH. These secretagogues have been shown to require the presence of GHRH and are synergistic with GHRH in promoting GH secretion. However, this has not been extensively studied.
Smith et al47 have described the secretagogue MK-677, a spiropiperidine that can be administered orally. It increases pulsatile GH release and serum IGF-I levels when administered by the oral route. This product fulfills the expectation of an agent mimicking as close as possible the in vivo effect of endogenous agents. Chapman et al48 investigated the use of this secretagogue in a randomized double-blind placebo-controlled trial involving 32 healthy elderly men and women. They found that once-daily treatment of oral MK-677 for 4 weeks enhanced pulsatile GH release, significantly increased serum GH and IGF-I concentrations, and, at a high dose of 25 mg daily, increased IGF-I levels to that of a young adult. The use of MK-677 alone or in combination with alendronate in the management of osteoporosis was studied in a small sample of 18 postmenopausal women with osteoporosis. The authors found that MK-677 enhanced the effect of alendronate on bone mineral density in the femoral neck but not lumbar spine and total hip.49 Side effects included fluid retention, increased serum transaminase, and serum glucose in some of the patients.43
GHRH has been studied in elderly men and women. IGF-I levels increased without any significant side effects. The metabolic effects were similar to that following GH administration. However, the drawback of this modality is again cost and the need for injections.
Ghrelin is known to be an even more potent stimulator of GH release than GHRH.1 Clinically this offers another therapeutic possibility in managing the aging decline of GH. Ghrelin may be administered as are the other GHSs to enhance the GH–IGF-I axis in healthy aging patients.13,14
What of the Future?
In this era of molecular medicine the prospects of research are exciting. In addition to the 191-amino-acid native peptide, the pituitary secretes other forms of GH. It would be ideal for an analog of GH that stimulates growth but lacks other actions of GH to be developed. It is tempting to speculate that the molecule of GH can be manipulated biochemically, and the altered GH molecule can be used to treat specific age-related problems provided that relevant end points have been defined.
Discussion of the Case History
Mr. J.M. is a somewhat typical person seeking growth hormone replacement. By and large, these individuals are healthy and have the resources, as the treatment is expensive, especially in the United States. It is not covered by insurance or Medicare. These patients tend to be well informed and rely much on the Internet for the latest developments in this area. Although the patient tested low on his IGF-I level, there was no dynamic testing done by the prior physician. The patient was advised that the exercise that he was doing was probably the best stimulator of growth hormone, and that it was wise to taper off the injectable growth hormone. On repeat testing, it was found that his IGF-I level was 402ng/mL (normal: 90–360) and was in the supraphysiological level range. He was advised about the side effects of growth hormone therapy and also that the long-term scientific literature at this point was scant. However, he was adamant on continuing the therapy, as he has noticed the loss of his body fat and improved quality of life. Informed consent was discussed at length. Counseling was provided and the patient was also advised about other options including plastic surgery. A compromise was reached, and the dose of growth hormone was reduced to 8 IU per week. The patient requested a discussion on GH-releasing peptides, nonpeptidyl secretagogues, and GHRH, and he continues to be followed up.
Conclusion and Key Points
The worldwide population is aging rapidly. Between 2000 and 2050, the population of people over 65 years of age is expected to double from 6.9 to 16.4%.50 There exists now an altered population structure with a longer life expectancy unmatched by health expectancy. This phenomenon has been largely brought about by increased life expectancy and lower fertility rates. For the individual, to live longer without quality of life is of little benefit, as advancing age brings with it increasing frailty and increasing susceptibility to illnesses. This has led to a search for agents and solutions to mitigate the continuous, universal, progressive, intrinsic, and deleterious process, which is aging. Growth hormone is one agent that has generated much excitement, passion, and controversy.
There is no doubt that GH is very important in body composition and metabolism. From a conservative viewpoint, low levels of GH associated with aging can be taken as a natural phenomenon and accepted as it is. On the other hand, if one takes health as a state of complete physical, social, and mental well-being, then a case can be made for restoring what has declined, to improve the quality of life of the aging individual. “Primum non nocere”: as physicians we must first do no harm. We must have no delusions that GH is the panacea for the aging individual. A circumspect and holistic approach is required. Lunenfeld’s51 advice to “prevent the preventable and delay the inevitable” does not start in the autumn of one’s life but in the spring. Although we cannot change our genetic destiny, we can at least be wise in the way we live, thereby reducing the negative impact of lifestyle-related diseases.
• It is established that there is a decline of GH with aging.
• The apparent benefits associated with GH therapy in healthy aging individuals seen in existing studies cannot yet be used to justify large-scale interventions among a healthy aging population.
• Large randomized, placebo-controlled studies with defined relevant end points will increase existing knowledge.
• GH secretagogues hold promise in the management of patients with age-related decline of GH, but more work has yet to be done.
• A thorough discussion of the pros and cons of GH replacement is essential, and informed consent is compulsory.
REFERENCES
1. Rosen T, Johannsson G, Johansson J, Bengtsson B. Consequences of growth hormone deficiency in adults and the benefits and risks of recombinant growth hormone treatment. Horm Res 1995;43:93–99
2. Savine R, Sönksen P. Growth hormone—hormone replacement for the somatopause? Horm Res 2000;53:37–41
3. Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med 1990; 323:1–6
4. Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res 2002;54:25–35
5. Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia: WB Saunders; 2000
6. Baumann G, Stalor MW, Amburn K, et al. A specific growth hormone binding protein in human plasma: initial characterization. J Clin Endocrinol Metab 1986;62:134–141
7. Greenspan FS, Gardner DG. Basic and Clinical Endocrinology. New York: Lange Medical Books/McGraw-Hill; 2001
8. Ho KY, Evans WS, Blizzard RM, et al. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 1987;64:51–58
9. Holl RW, Hartman ML, Veldhuis JD, et al. Thirty-second sampling of plasma growth hormone (GH) in man: correlation with sleep stages. J Clin Endocrinol Metab 1991;72:854–861
10. Wideman L, Weltman JY, Hartman ML, et al. Growth hormone release during acute and chronic aerobic and resistance exercise: recent findings. Sports Med 2002;32:987–1004
11. Ehrnborg C, Lange KHW, Dall R, et al. The growth hormone/insulin-like growth factor-1 axis hormones and bone markers in elite athletes in response to a maximum exercise test. J Clin Endocrinol Metab 2003;88:394–401
12. Cappon J, Brasel JA, Mohan B, et al. Effect of brief exercise on circulating insulin-like growth factor-1. J Appl Physiol 1994;76: 2490–2496
13. Petersenn S. Growth hormone secretagogues and Ghrelin: an update on physiology and clinical relevance. Horm Res 2002; 58:56–61
14. Kojima M, Hosoda H, Matsuo H, et al. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab 2001;12:118–126
15. Martin FC, Yeo A, Sonksen PH. Growth hormone secretion in the elderly: aging and the somatopause. Baillieres Clin Endocrinol Metab 1997;11:223–250
16. Clemmons DR, Van Wyk JJ. Factors controlling blood concentration of somatomedin C. J Clin Endocrinol Metab 1984;13: 113–143
17. Gooren L. Age-related decline of growth factor hormone (somatopause). In: Gooren L, Lim PHC, eds. The Aging Male. Singapore: MediTech Media Asia Pacific; 2001:33–41
18. Holly JMP, Wass JAH. Insulin-like growth factors; autocrine, paracrine or endocrine? new perspectives on the somatomedin hypothesis in the light of recent developments. J Endocrinol 1989;122:611–618
19. Guler HP, Zapf J, Schmid C, et al. Insulin-like growth factors I and II in healthy men: estimations of half-lives and production rates. Acta Endocrinol (Copenh) 1989;121:753–758
20. Rudman D, Kutner MH, Rogers CM, et al. Impaired growth hormone secretion in the adult population. J Clin Invest 1981; 67:1361–1369
21. Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative obesity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 1991;73:1081–1088
22. Zadik Z, Calew SA, McCarter RJ, et al. The influence of age on the 24-hour integrated growth hormone concentration in normal individuals. J Clin Endocrinol Metab 1985;60:513–516
23. Corpas E, Hartman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev 1993;14:20–39
24. Reutens AT, Veldhuis JD, Hoffman D, et al. A highly sensitive growth hormone (GH) enzyme-linked immunosorbent assay uncovers increased contribution of a tonic mode of GH secretion in adults with organic GH deficiency. J Clin Endocrinol Metab 1996;81:1591–1597
25. Toogood AA, O’Neill PA, Shalet SM. Beyond the somatopause: growth hormone deficiency in adults over the age of 60 years. J Clin Endocrinol Metab 1996;81:460–465
26. Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, et al. Serum insulin-like growth factor 1 in a random population of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf) 1994;41:351–357
27. Rudman D, Mattson DE. Serum insulin-like growth factor-I in healthy older men in relation to physical activity. J Am Geriatr Soc 1994;42:522–527
28. Jørgensen JOL, Troels KH, Flavia LC, et al. Somatopause and body composition. In: Lunenfeld B, Gooren L, eds. Textbook of Men’s Health. London: Parthenon Publishing Group; 2002
29. Vahl N, Jorgensen JOL, Jurik AG, et al. Abdominal adiposity and physical fitness are major determinants of the age associated decline in stimulated GH secretion in healthy adults. J Clin Endocrinol Metab 1996;81:2209–2215
30. Consensus Guidelines for the Diagnosis and Treatment of Adults with Growth Hormone Deficiency: Summary Statement of the Growth Hormone Research Society Workshop on Adult Growth Hormone Deficiency. J Clin Endocrinol Metab 1998;83:379–381
31. Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med 1990; 323:1–6
32. Thompson JL, Butterfield GE, Marcus R, et al. The effects of recombinant human insulin-like growth ractor-I and growth hormone on body composition in elderly women. J Clin Endocrinol Metab 1995;80:1845–1852
33. Cohn L, Feller AG, Draper MW, et al. Carpal tunnel syndrome and gynecomastia during growth hormone treatment of elderly men with low circulating IGF-1 concentrations. Clin Endocrinol (Oxf) 1993;39:417–425
34. Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in older men improves body composition but not functional ability. Ann Intern Med 1996;124:708–716
35. Blackman MR, Sorkin JD, Munzar T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 2002;288:2282–2292
36. Goh VHH, Mu SC, Gao F, et al. Changes in body composition and endocrine and metabolic functions in healthy elderly Chinese men following growth hormone therapy. The Aging Male 1998; 1:264–269
37. Geusens PP, Boonen S. Osteoporosis and the growth hormone-insulin-like growth factor axis. Horm Res 2002;58:49–55
38. Holloway L, Butterfield G, Hintz R, et al. Effects of recombinant human growth hormone on metabolic indices, body composition and bone turnover in healthy elderly women. J Clin Endocrinol Metab 1994;79:470–479
39. Aloia JF, Zanzi I, Ellis K, et al. Effects of growth hormone in osteoporosis. J Clin Endocrinol Metab 1976;43:992–999
40. Yarasheski KE, Zachwieja JJ, Campbell JA, et al. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol 1995;268:E268–E276
41. Pagana KD, Pagana TJ. Mosby’s Manual of Diagnostic and Laboratory Tests. St. Louis: Mosby; 1998
42. Blackman MR. Growth hormone and aging in men. In: Lunenfeld B, Gooren L, eds. Textbook of Men’s Health. London: Parthenon Publishing Group; 2002
43. Khorram O. Use of growth hormone and growth hormone secretagogues in aging: help or harm. Clin Obstet Gynecol 2001; 44:893–901
44. Vance ML. Can growth hormone prevent aging? N Engl J Med 2003;348:779–780
45. Taaffe DR, Pruitt L, Reim J, et al. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab 1994; 79:1361–1366
46. Hurel SJ, Koppiker N, Newkirk J, et al. Relationship of physical exercise and aging to growth hormone production. Clin Endocrinol (Oxf) 1999;51:687–691
47. Smith RG, Cheng K, Schoen WR, et al. A nonpeptidyl growth hormone secretagogue. Science 1993;260:1640–1643
48. Chapman IM, Bach MA, Van Cauter E, et al. Stimulation of the growth hormone (GH)-insulin-like growth factor I axis by daily oral administration of a GH secretagogue (MK-677) in healthy elderly subjects. J Clin Endocrinol Metab 1996; 81:4249–4256
49. Murphy MG, Weiss S, McClung M, et al. Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab 2001;86:1116–1125
50. Department of International Economic and Social Affairs. Periodical on Aging. New York: United Nations; 1985;1:1–61
51. Lunenfeld B. Healthy aging for men. Climacteric 1999;2:9
< div class='tao-gold-member'>









