6
Depression in Aging Men: Are There Unique Gender-Based Therapies?
Case History
A 62-year-old man with a long history of depression has had multiple hospitalizations for alcohol abuse and depression and has received a diagnosis of bipolar II disorder after a history of occasionally experiencing hypomanic symptoms. He is currently taking Wellbutrin and hydroxyzine. He claims that antidepressant therapy and abstinence from alcohol have somewhat helped in reducing his depressive symptoms, but he still generally lacks interest, energy, libido, and desire for pleasurable activities. He states that he is often unmotivated to work, and he fears for the future of his business because of this. He has been aware of his low testosterone level for a couple of years and has tried dehydroepiandrosterone (DHEA) and other various methods in an attempt to boost his sex drive and energy level. For several years, he has had frequent difficulty falling asleep despite trying several different sleeping aids. Examination revealed that he weighed 263.4 pounds, indicating moderate obesity; blood pressure (BP) was 161/88 mm Hg. His 5 A.M. total testosterone was 210ng/dL, and his prostate-specific antigen (PSA) 0.38ng/mL. He scored in the moderate range (17) on the 21-item Hamilton Rating Scale for Depression (HAM-D) and endorsed a poor to fair level of life satisfaction on the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q).
The patient was enrolled in a double-blind study during which he received placebo for the first 12 weeks and testosterone for the second 12 weeks. During what was later discovered to be the placebo condition, his mood and energy level became increasingly worse. The date was approaching a trauma-related anniversary, and he claimed that his depression often worsened at that time of year. He also reported more difficulty sleeping, claiming that none of the previous medications were working. He was very irritable and easily aggravated, and he was sure he was being given a placebo. After 12 weeks of the initial treatment, he was crossed over to receive testosterone.
When contacted shortly after beginning testosterone treatment, he had noticed a dramatic increase in his mood and energy level. He stated that within 3 hours he began to feel “normal” as if a “fog had been lifted.” His sleep and sex drive had also improved, although not as much as his depression. After 6 weeks of testosterone treatment, he reported depressive symptoms in the mild range (11) on the HAM-D, and after 12 weeks of treatment, his depression had improved even more (7), and ratings on the Q-LES-Q indicated increased life satisfaction. Laboratory results at 12 weeks concluded that his total testosterone had risen to 1060 ng/dL.
Epidemiological Perspectives of Depression in Aging Men
Overall, depression affects at least 3 million to 4 million men in the United States.1 It has been estimated that one out of every 10 men will be diagnosed with depression in his lifetime.2 Studies suggests that ∼8 to 16% of community-dwelling seniors have significant depression.3,4 However, the frequency of depression in the institutional setting such as in long-term care can reach as high as 20%.5,6 Based on community studies, it is generally believed that men are less likely to suffer from depression than women. However, the community-based data may be skewed, as men are less likely to report symptoms. It is of significance that the prevalence rate of depression in institutionalized men equals that of women, especially if severity of illness and functional impairment are controlled.5,6 Men are more likely than women to view a diagnosis of depression as a sign of weakness and therefore are less likely than women to seek treatment.2
However, the impact of depression on men seems to be greater with more tragic consequences. For instance, the rate of suicide in men is four times higher than women. More women than men attempt suicide but often do not complete suicide.7 Many studies have also established that older white men are particularly prone to a risk of suicide.8 Older men are also more likely to complete suicide, and resort to violent means such as a shooting or hanging themselves. The marital status of a man is also a factor for suicide. For instance, widowed and divorced men have the highest rates of suicide.9 Younger widowed men are also very likely to kill themselves. It has been hypothesized that loneliness, comorbid physical illness, alcohol abuse, and impulsive behaviors can be contributing factors in these suicide attempts.
The physical impact of depression in men is also different from that in women. Depression can affect a man’s physical health differently from how it might affect a woman’s. A recent study showed that both men and women who suffer from depression are at an increased risk of coronary heart disease; however, only men suffer a higher rate of death.10 Men are more likely to deal with depression by turning to drugs and alcohol or by being workaholics.1 Depression may have atypical presentations in men. Symptoms like irritability, anger, and discouragement can occur because of depression, and depression can overlap with clinical situations including hypogonadism (andropause), thyroid disorders, and alcohol and substance abuse.
Etiological Perspectives of Depression in Older Men
In general, the causes of late-life depression can be divided into biological, psychological, and social factors.11 Biological factors include hereditary factors, neuroanatomical changes, neurotransmitter abnormalities, dysregulation of endocrine function, and dysregulation of circadian rhythms, especially sleep.
Biological Factors
In considering hereditary factors, there is currently no evidence that older men are at greater or lesser risk for depression secondary to genetic factors than older women.12 Previously, it had been postulated that women may be at greater risk than men for depression secondary to genetic factors, and men may be at greater risk than women for alcoholism secondary to genetic factors. This hypothesis has been expanded to suggest that men and women may carry the same gene, which expresses itself as alcoholism in men and depression in women. The notion that women may be more affected by genetics has also been suggested by assumptions that the gene for depression may be carried on the X chromosome and transmitted as an X-linked dominant gene.13 Evidence at present, however, does not support an X-linked dominant transmission.
Thirty percent of persons older than age 60 can exhibit patchy deep white matter lesions of abnormal signal intensity with magnetic resonance imaging (MRI).14 These lesions have been associated with hypertension, and are noted more frequently among men.15 It has also been noted that these lesions are commonly found in cases of clinical depression. Some investigators have suggested that these lesions may define a unique type of depression in the elderly, called vascular depression.15 In general, patients with vascular depression are more likely to experience apathy. Vascular depression, if diagnosed, is more prevalent in men, but the costs of MRI often limit diagnosing this entity, which may be more common among older men.
A second major biological contribution to depression is neurotransmitter abnormalities, including, but not exclusively, the serotonin neurotransmitter system. Investigators have found reduced cerebrospinal fluid levels of 5-hydroxyindoleacetic acid in patients with depression. Research does not suggest neurotransmitter depletion or dysfunction to be more prominent among older persons than younger persons. In addition, there are no gender differences in neurotransmitter function.16 Elevated levels of cortisol, which is arguably a biological indicator for stress, is associated with depression. However, no studies have suggested a gender difference in hypothalamic-pituitary-adrenal (HPA) axis functioning, baseline cortisol levels, or response of the HPA axis to stimulation with dexamethasone.
Psychological and Social Factors
According to cognitive and behavioral theories, depression results from three specific processes: the negative triad, underlying beliefs or schemas, and cognitive errors. An interactive set of negative views toward the self, experience, and the future defines the negative triad. As such, psychological factors may impact depression in men as well. Under this belief, psychotherapists work to relieve depression through psychotherapy. Social factors are also major contributors to depression in late life. Examples of stressful life events that can lead to depression in older men include death of a spouse, sudden onset of a physical illness, and retirement. Chronic stress can include situations that challenge or threaten the older man’s well-being. Examples of chronic stress also include poverty, ongoing interpersonal difficulties, or living in a dangerous neighborhood. Daily hassles are the usual yet stressful events and interactions experienced by older persons on a regular basis. Examples of daily hassles include managing household finances, home maintenance, and unpleasant interactions with neighbors. Each of these may contribute significantly to depression in older persons. Impaired social support networks have been implicated as contributing to depression.17 Social support can contribute to depression, but may vary by gender. In one study, women but not older men were sheltered against the impact of stressful life events by adequate social support.18 Men are generally thought to have fewer supports available to them. Although men are more likely to live in a household with someone else, they typically report having fewer friends whom they see on a regular basis. Having fewer available supports contributes to the possibility of an etiological risk factor for depression in older men.
Diagnosis of Depression in Men
Men are less likely than women to report a depressed mood or to conceive of their dysphoric state as being associated with a psychiatric illness.19 Direct questioning may sometimes elicit the diagnosis. The older man can have atypical presentations for depression, and can report insomnia, loss of appetite, lethargy, fatigue, difficulty concentrating, and even anger. Medical conditions like hypothyroidism, hypogonadism, and anemia can also have similar presentations. Sometimes patients report having thoughts that life is not worth living, but they rarely report overt thoughts of suicide. If the older man admits that he has considered taking his life, the clinician must deem this person at significant risk for suicide and take action accordingly. As this is a psychiatric emergency, hospitalization may be required until the risk of suicide diminishes with treatment.
In the severely depressed older man, laboratory studies are important, not so much for the diagnosis of depression as for the identification of secondary medical abnormalities that may either accompany or derive from the depressed mood. Therefore, the clinician should order a complete blood count, urinalysis, blood chemistry, thyroid profiles, and a drug screen. Testosterone levels can be measured if clinical hypogonadism is suspected, as low testosterone may be associated with depression. A baseline electrocardiogram (ECG) should be obtained, for two reasons: medications such as tricyclics may alter cardiac rhythms, and the depressed older man is often more likely to suffer concomitant heart disease. If the older man suffers from cognitive dysfunction along with the depressed mood, a workup for dementia should be performed.
Standardized Acceptable Treatments for Depression in Men
As the etiology of depression is divided into biological, psychological, and social components, treatments should also be directed to these components. Physicians often focus on the biological aspects of treatment and neglect the other components because of time and reimbursement issues. Nevertheless, effective treatment must include each of these components. Biological treatments include pharmacological therapy and electroconvulsive therapy (ECT).
The old adage of “start low, go slow” applies to the treatment of depression in older men as well. In general, the treatment should commence with an antidepressant medication such as selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine (Prozac), paroxetine (Paxil), and sertraline (Zoloft). In general, SSRIs, as compared with tricyclic antidepressants, are equally valuable but produce fewer side effects during treatment. Also, issues of overdosing are less consequential with SSRIs. Sometimes, tricyclic antidepressants can be prescribed in the first instance, as when, for example, the patient on SSRIs complains of persistent nausea and vomiting, resulting in weight loss. When starting an antidepressant, the axiom has been “start low, go slow.” For example, in elderly men, starting doses can be nortriptyline 50 mg/day and desipramine 50 mg/day A tetracyclic antidepressant, like trazodone or nefazodone, can be effective. Tetracyclic antidepressants can be especially useful for agitated depression and depression with insomnia symptoms. In contrast to the SSRIs, both the tricyclic antidepressants and tetracyclics require increasing doses over time.
To ensure compliance, the clinician must pay special attention to the side effects of SSRIs. Unfortunately, one of the more common long-term side effects of SSRIs is sexual dysfunction. Older men may experience great concern regarding the loss of sexual function but yet not discuss this with the doctor. It is important to forewarn patients of this potential side effect. SSRIs may also decrease the appetite of the older man. Weight loss, even 5 pounds, can be most disturbing and problematic in the treatment of frail older men. If these medications amplify the potential for weight loss, they should be discontinued and replaced by tricyclic antidepressants, which paradoxically may potentiate weight gain. Another known side effect of SSRI is agitation. All of the SSRIs can produce agitation, but fluoxetine is most likely to do so. Also, fluoxetine has a long half-life, and this side effect may not arise until the drug has been taken over several days. There is no evidence to suggest that the SSRIs increase the risk of suicide in older men. However, severe agitation is a risk for suicide. Careful monitoring by the clinician of these medications is therefore essential.
Space limitations prevent us from discussing all the new antidepressants that have been marketed in recent years. The important ones will be mentioned briefly.20 Venlafaxine (Effexor) is a structurally novel compound first approved by the Food and Drug Administration (FDA) in 1993 for the treatment of major depression. It is a bicyclic antidepressant that produces strong inhibition of both norepinephrine and serotonin. Initially, venlafaxine was released in an immediate-release (IR) form that is taken two or three times daily. However, in 1997, an extended-release form (Effexor XR) was approved by the FDA, which allowed for a once-a-day administration. The recommended venlafaxine XR starting dosage is 37.5 to 75 mg per day. The dosage may be increased in increments of up to 75 mg every 4 to 7 days, to a maximum daily dosage of 225 mg. Venlafaxine is the first antidepressant that has proved effective in treating patients with generalized anxiety disorder (GAD). The recommended dosage is similar to that in depression. Some patients can experience jitteriness with the usual starting dose of 75 mg per day, so beginning treatment at a dose of 37.5 mg per day for the first week is advisable. Overall, the side-effect profile is comparable to that of the SSRIs and lower than that of the tricyclic antidepressants. The most common side effects include nausea, dizziness, insomnia, somnolence, and dry mouth. Anticholinergic side effects are significantly less severe than those encountered with other antidepressants, and thus this medication is more suitable for older patients. Sexual side effects are similar to those caused by SSRIs.
Mirtazapine (Remeron) is a tetracyclic antidepressant unrelated to tricyclic antidepressants and SSRIs. It is unique in its action among the currently available antidepressants. Mirtazapine is a presynaptic α2-adrenergic receptor antagonist plus a potent antagonist of postsynaptic 5-hydroxytryptamine 5-HT2 and 5-HT3 receptors. The net outcome of these effects is stimulation of the release of norepinephrine and serotonin. Current evidence suggests that mirtazapine is effective in the treatment of all levels of depressive illness. In addition, analyses of placebo-controlled trials in moderate and severe depression have shown mirtazapine to be effective in subgroups of depressed patients, particularly those with anxiety, sleep disturbance, and agitation, as well as mentally retarded patients. Mirtazapine has an onset of efficacy of 2 to 4 weeks. However, sleep disturbances and anxiety symptoms may improve in the first week of treatment. In a review of multiple double-blind studies comparing mirtazapine with SSRIs, the proportion of responders with onset of persistent improvement in week 1 was twice as great with mirtazapine (13% versus 6%).
Mirtazapine’s unique pharmacological profile is virtually devoid of anticholinergic, adrenergic, and serotonin-related side effects. The most frequently reported adverse events were fatigue, dizziness, transient sedation, and weight gain. Sexual dysfunction is not a side effect of this agent. Drug interactions with mirtazapine have not been studied systematically. The recommended starting dosage is 15 mg at bedtime, which may be titrated up to 45 mg daily, if needed.
Although by now a fairly well-established antidepressant, bupropion (Wellbutrin) warrants mention based on its increasing use in the augmentation of SSRIs. Uses include a role as a possible antidote for SSRI-induced sexual dysfunction, as a favored agent for bipolar depression, as a stimulant-like agent that can produce beneficial cognitive and behavioral effects in pediatric and adult attention deficit hyperactivity disorder, and, more recently, because of its newly marketed repackaging as an antismoking aid. Bupropion, under the name Zyban, received FDA approval for smoking cessation. A sustained release form is now commonly prescribed (150 mg b.i.d., with a maximal dose of 200 mg b.i.d.). Bupropion apparently causes the least amount of sexual dysfunction in the treatment of sexual dysfunction.21
If the older depressed man does not respond to antidepressant medications and exhibits severe symptoms, ECT may be indicated. As not all psychiatrists are trained in ECT or have access to these facilities, the patient may need to be referred elsewhere. Today, ECT can be performed in outpatient settings. In general, ECT is used more often in treating older men than younger men because of the increased resistance to medications in older men. Also, psychotic depression is more frequent in late life, and this variant of depression is often responsive only to ECT. Although the response rate to ECT is excellent, frequently exceeding 80%, the relapse rate in many studies has been greater than 50%. By continuing treatments for weeks and even months at infrequent intervals, the relapse rate decreases dramatically.
Finally, psychotherapy can play an integral role in the treatment of moderate-to-severe depression in the older man. In general, there are two types of psychotherapy: cognitive-behavioral therapy and interpersonal therapy. However, time and reimbursement issues have made psychotherapy impractical in many clinical settings, particularly so in primary care. There is not much specific research on gender-based psychotherapy methods, but it is well known that psychotherapy in combination with medications can be additive in its effect.
Low Endogenous Testosterone and Depression
Often hypogonadal males exhibit significant psychiatric symptoms, including dysphoria, fatigue, irritability, and appetite loss, and these symptoms are generally alleviated after testosterone replacement. Davidson et al22 found no effect on mood states when giving men with hypogonadism, age 32 to 65 years, two doses of testosterone enanthate (TE) (100 and 400 mg per month) for 5 months. By contrast, other studies have shown that administering TE improved mood.23–26 Eight subjects recorded their mood states daily in diary format and completed visual analog scales rating 10 separate mood states. Significant TE-related improvements were found for several scales, including cheerful-happy, tense-anxious, and relaxed.23 Burris and colleagues,24 using self-reported measures, found that six hypogonadal men (age range 25–40 years) scored higher on ratings of depression, anger, fatigue, and confusion than did controls. After 200 mg every 2 weeks of TE treatment, mood scores improved, suggesting testosterone treatment had some capacity to elevate mood and decrease anger. In a similar study, Wang et al25 studied 58 men (age range 22–60 years) with hypogonadism who were treated with either 200 mg of TE every 20 days or sublingual testosterone cyclodextrin (T) at 2.5 or 5mg three times a day for changes in mood. These researchers concluded, after T replacement therapy for 60 days, that T treatment led to increased energy, good feelings, and friendliness, and decreased anger, nervousness, and irritability. Similarly, mood and energy improved with transdermal T administration using scrotal patches.26
Androgen replacement also has been applied to men with low T levels associated with Klinefelter’s syndrome. No reported mood or energy differences were reported for four men given 160 mg testosterone undecanoate (TU) or placebo in a double-blind crossover study.27 In a larger trial, 30 men with Klinefelter’s syndrome were given T.28 Follow-up telephone interviews revealed improved mood, less irritability, more energy and drive, less tiredness, more endurance and strength, less need for sleep, and better concentration.
Some studies report no difference in T levels between depressed and nondepressed men.29,30 However, others have reported differences. For example, Vogel et al31 reported that in 27 men with unipolar depression, total and free T were significantly lower by 30% than in 13 age-matched controls. Rubin et al32 studied the hypothalamic-pituitary-gonadal (HPG) axis in 16 depressed men and 16 healthy age-matched controls. They found a statistically significant negative correlation between age and total T levels in depressed patients, suggesting that in depressed men the decline in total serum T is correlated with advancing age and depression. Davies et al33 found a significant negative correlation between salivary T levels and severity of depression in 11 men with a mean age of 52.4 years. In summary, some men with major depression may have significantly lower free and bioavailable T than their age-matched controls. Also, the levels are significantly lower as subjects age, and probably lower with greater severity of depression.
The Rancho Bernardo Study, a cross-sectional population-based study, examined the association between endogenous sex hormones and depressed mood in community-dwelling older men.34 Figure 6–1 shows the relationship between low testosterone and depression. The study found that free T decreases with age, and the prevalence of depressive symptoms increases with age. Analyses showed that there was a stepwise decrease in free T with increasing levels of depressed mood, independent of age, weight change, and physical activity.
Plausibility of Adjuvant Testosterone Therapy for Depression
Adjuvant testosterone administration has been used experimentally to treat patients with well-diagnosed depression. The antidepressant effect of a synthetic weak androgen, mesterolone was compared with amitriptyline in men with depression.35 Mesterolone relieved depression as effectively as amitriptyline. Additionally, mood enhancement was also found with the weak androgen dehydroepiandrosterone.36
Rabkin et al37 have conducted two studies of T administration to HIV-positive men. In the first, an open clinical trial of 44 hypogonadal HIV-positive men who had mood problems and completed an 8-week trial of intramuscular T, 28 (64%) were much improved compared with baseline. In the second study, 74 HIV-positive men were enrolled in the double-blind, placebo-controlled, 6-week trial with biweekly testosterone versus placebo, followed by 12-week open-label treatment.38 Based on Clinical Global Impression (CGI) Scale ratings, response to testosterone was 74%, and to placebo was 19% (p <.001). Among 26 study completers with Axis I depressive disorders, 58% of those randomized to testosterone versus 14% of placebo-treated patients were judged to have much or very much improved mood. There was no difference in response rate between men with T levels below versus within normal range. Grinspoon et al39 also showed that hypogonadal men with AIDS wasting had increased depression scores. In these patients, administration of testosterone (300 mg intramuscularly [IM] every 3 weeks) resulted in significant improvement of depression inventory scores (Fig. 6-2).
Intramuscular testosterone was successfully used as the only treatment of a suicidal depression in a case of bilateral cryptorchidism29 and in two cases of depression associated with Klinefelter’s syndrome.40 Seidman and Rabkin41 administered testosterone openly to five men who had SSRI-refractory major depression and T level below 350 ng/dL. In the 8-week trial, all five patients had rapid dramatic recovery, with mean HAM-D decrease from 19.2 to 7.2 by week 2, and to 4.0 by week 8. This was an open trail with a small number of subjects. Seidman et al42 also completed a randomized, placebo-controlled clinical trail examining the effects of testosterone enanthate 200mg IM or placebo administration in 32 men with major depressive disorder, as defined in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), and a low testosterone level. The authors found that the HAM-D score decreased in both the 13 subjects receiving testosterone and in the 17 subjects receiving placebo. There were no significant between group differences.
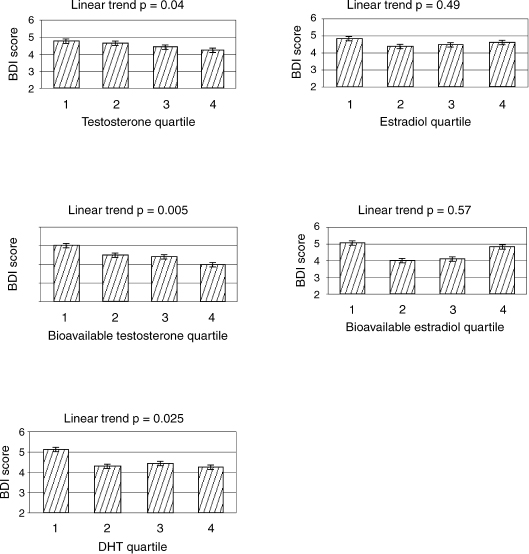
FIGURE 6-1. Data from the Rancho Bernardo study showing relationship of low testosterone to depression. BDI, Beck Depression Index. (Adapted from Barrett-Connor E, von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo study. J Clin Endocrinol Metab 1999;84:573–577, with permission.)
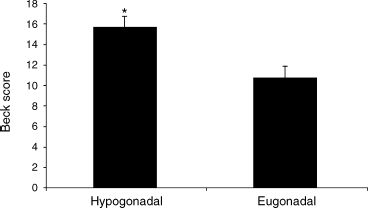
FIGURE 6-2. Human immunodeficiency virus (HIV) patients who are hypogonadal have higher BDI scores. (Adapted from Grinspoon S, Corcoran C, Stanley T, Baaj A, Basgoz N, Klibanski A. Effects of hypogonadism and testosterone administration on depression indices in HIV-infected men. J Clin Endocrinol Metab 2000;85:60–65, with permission.)
Perry et al43 recently completed a 6-week randomized study with an initial single-blind 2-week placebo period evaluating the efficacy of testosterone therapy for 16 elderly eugonadal men with major depressive disorder (DSM-IV criteria) and HAM-D scores >18. Patients received testosterone cypionate in either a physiological dose of 100 mg/week or a supraphysiological dose of 200 mg/week. Results indicated an improvement in HAM-D scores in both groups, although the majority of the change was due to improvement in late-onset (≤ 45 years old) depression patients.
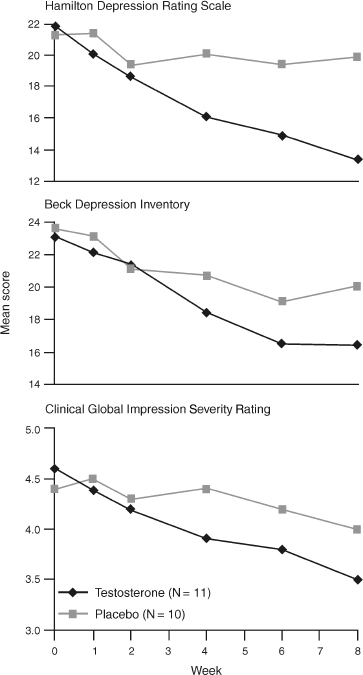
FIGURE 6-3. Effects of testosterone against placebo in treating depression. (Adapted from Pope HG, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105–111, with permission.)
In another recent randomized, placebo-controlled trial, Pope et al44 assigned 23 subjects to complete a 1-week single-blind placebo period, and then randomly assigned 22 subjects to receive 10 g testosterone gel or placebo for 8 weeks. Subjects included men aged 30 to 65 currently on antidepressant therapy with refractory depression and morning serum total testosterone levels of 350 ng/dL or less. Subjects receiving testosterone had significantly greater improvement in overall HAM-D scores and the vegetative and affective subscales of the HAM-D than did subjects in the placebo group (Fig. 6–3).
In summary, T supplementation may improve mood in patients with depression and/or hypogonadism. Additional work that is focusing on T supplementation and depressive disorder type and depth will likely elucidate the apparent interaction.
The Influence of Stress as a Confounder in Hypogonadism and Depression
Can stress lead to temporary or permanent suppression of testosterone production? It is highly likely, but still speculative, although it has at least been observed in clinical settings. However, it can be stated that in general, the endocrine response to stress is complex.45 Elevations in the serum concentrations of the classic stress hormones epinephrine and cortisol occur following many kinds of physiological challenge and are accompanied by elevations in corticotropin, growth hormone (GH), and glucagon levels. These changes are probably responsible for the hyperglycemia and hypercatabolism common to most critical illness. In addition, if volume depletion is present, vasopressin, renin, and aldosterone secretion are also likely to be stimulated. These hormones, if present in excess, may produce fluid retention and hyponatremia. We are aware that thyroid hormone metabolism is commonly affected by critical illness, which results in characteristic abnormalities of thyroid function testing known as the euthyroid sick syndrome.
The reproductive axis is also exquisitely sensitive to physiological stress; hypogonadotropic hypogonadism is a common finding in stress situations. In females, it is well established that stress can lead to menstrual abnormalities including amenorrhea. There is not much scientific literature on the observation of depression of androgen production with stress. However, it has been reported that male endurance runners can acquire a hypogonadotrophic hypogonadal state.46 The mechanism whereby hypogonadism occurs is unclear and is postulated to be central. In space, where there is low gravity, temporary episodes of hypogonadism have been experienced in astronauts.47 It can be argued that microgravity puts a stress on these astronauts and hence the hypogonadal state. In practice, stress is often encountered in patients. The challenge to the clinician is to determine whether seemingly abnormal hormone measurements in these patients reflect an appropriate homeostatic response to stress or whether they indicate an independent metabolic disorder that might actually cause or contribute to the patient’s condition. The approach may be waiting and observing, as testosterone levels have been normalized in some patients without pharmaceutical interventions.
Discussion of Case History
This patient highlights the difficult area of the relationship between depression and hypogonadism. It can be argued in this case that he had clinical depression for many years, and that his hypogonadism added to his depressive feeling. It is unlikely that his depression was due to only his hyogonadal state. (In some instances, it could be the primary cause.) He was fortunate to have been crossed over and randomized to the drug during the clinical trial. It resulted in a dramatic increase in his mood and energy level. The added benefit was that his sexuality improved tremendously after androgen replacement. The placebo did not achieve the same effect for him. Alcohol abstinence also helped relieve his symptoms as well.
Conclusion and Key Points
Depression is a complex, multifaceted illness. It is sometimes a response to illness. Chronic illness in itself can suppress testosterone production. As a result, it is difficult to attribute depression to hypogonadism directly. Depression remains a huge public health issue, and has to be diagnosed before being treated. Many older men go undiagnosed, and this illness is confounded by multiple medical illnesses as well. There exists multiple screening tools for depression, and it is prudent for clinicians to spot depression in their interviews, as nontreatment can have tragic consequences.
• Community studies suggest a lower prevalence of depression in men than in women, but the data may be skewed as men in general report symptoms less frequently.
• The impact of depression is greater in men, as suicides tend to be more frequent. As such, screening for depression in men is important.
• There can be atypical presentations of depression in men, and it can include anger and irritability.
• There is no evidence of either genetics or neurotransmitter differences in depression. However, lack of social support in men can result in tragic outcomes in depressed men.
• As pharmacokinetics and pharmacodynamics are affected with aging, it is prudent to “start low and go slow” with antidepressants.
• Epidemiological studies support the finding of low testosterone and depression.
• Stress can result in lowering of hormones in men, including testosterone.
• Adjuvant testosterone therapy for depression in men has been studied, and results are encouraging but not conclusive. As such, standard anti-depressives should always be used as first-line therapy.
• There may be a place for adjuvant testosterone therapy in the patient who is depressed and has concomitant hypogonadism.
REFERENCES
1. National Institute of Mental Health. Depression. NIMH publication No. 00–3561. Bethesda, MD: NIMH, 2000
2. Blehar MD, Oren DA. Gender differences in depression. Medscape Women’s Health 1997;2:3. Revised from: Women’s increased vulnerability to mood disorders: integrating psychobiology and epidemiology. Depression 1995;3:3–12
3. Blazer D, Hughes DC, George LK. The epidemiology of depression in an elderly community population. Gerontologist 1987;27:281–287
4. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med 1988;18: 141–153. Erratum in Psychol Med 1988;18:following 792.
5. Koenig HG, Meador KG, Cohen HJ, et al. Depression in elderly hospitalized patients with medical illness. Arch Intern Med 1988;148:1929–1936
6. Parmelee PA, Katz IR, Lawton MP. Depression among institutionalized aged: assessment and prevalence estimation. J Gerontol 1989;44:M22–M29
7. Meechan P, Salsman L, Satin R. Suicide among older United States residents: epidemiologic characteristics and trends. Am J Public Health 1991;81:1198–1200
8. Baldwin R, Jolley DJ. The prognosis of depression in old age. Br J Psychiatry 1986;149:574–583
9. Smith J, Mercy JA, Conn J. Marital status and the risk of suicide. Am J Public Health 1988;78:78–80
10. Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch Intern Med 2000;160:1261–1268
11. Blazer D. Depression and the older man. Med Clin North Am 1999;83:1305–1316
12. Rice J, McGuffin P. Genetic etiology of schizophrenia and affective disorders. In: Michels R, ed. Psychiatry. Philadelphia: JB Lippincott; 1990
13. Winokur G, Clayton P. Family history studies, II: sex differences and alcoholism in primary affective illness. Br J Psychiatry 1967;113:973–979
14. Bradley W, Waluch V, Brandt D, et al. Patchy, periventricular white matter lesions in the elderly: a common observation during NMR imaging. Noninvasive Medical Imaging 1984; 1:35–41
15. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry 1997;154:497–501
16. Bissette G. Chemical messengers. In: Busse E, Blazer D, eds. Textbook of Geriatric Psychiatry. Washington, DC: American Psychiatric Press; 1996:73–94
17. Landerman R, George L, Campbell R, et al. Alternative models of the stress buffering hypothesis. Am J Community Psychol 1989;17:625–642
18. George LK, Blazer DG, Hughes DC, et al. Social support and the outcome of major depression. Br J Psychiatry 1989;154:478–485
19. Garrard J, Rolnick SJ, Nitz NM, et al. Clinical detection of depression among community-based elderly people with self-reported symptoms of depression. J Gerontol Series A Biol Sci Med Sci 1998;53:M92–101
20. Ables AZ. Antidepressants: update on new agents and indications. Am Fam Physician 2003;67:547–554
21. Masand PS. Sustained-release bupropion for selective serotonin reuptake inhibitor-induced sexual dysfunction: a randomized, double-blind, placebo-controlled, parallel-group study. Am J Psychiatry 2001;158:805–807
22. Davidson JM, Camargo CA, Smith ER. Effects of androgen on sexual behavior in hypogonadal men. J Clin Endocrinol Metab 1979;48:955–958
23. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men—a clinical research center study. Clin Endocrinol 1985;23:527–538
24. Burris AS, Banks SM, Carter CS, Davidson JM, Sherins R. A long-term, prospective study of the physiologic and behavior effects of hormones replacement in untreated hypogonadal impotent men. J Androl 1992;13:297–304
25. Wang C, Alexander G, Berman N, et al. Testosterone replacement therapy improves mood in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab 1996;81: 3578–3582
26. Cunningham GR, Snyder PJ, Atkinson LE. Testosterone transdermal delivery system. In: Bhasin S, Gabelnick HL, Spieler JM, et al., eds. Pharmacology, Biology, and Clinical Applications of Androgen. New York: Wiley-Liss; 1996:437–447
27. Wu FCW, Bancroft J, Davidson DW, et al. The behavioural effects of testosterone undecanoate in adult men with Klinefelter’s syndrome: a controlled study. Clin Endorcrinol 1982;16: 489–497
28. Nielsen FH, Hunt CD, Mullen LM. Effect of dietary boron on mineral, estrogen and testosterone metabolism in postmenopausal women. FASEB J 1987;1:394–397
29. Levitt AJ, Joffe RT. Total and free testosterone in depressed men. Acta Psychiatr Scand 1988;77:346–348
30. Sachar EJ, Halpern F, Rosenfeld RS, Galligher TF, Hellman L. Plasma and urinary testosterone levels in depressed men. Arch Gen Psychiatry 1973;28:15–18
31. Vogel W, Klaiber EL, Broverman DM. Roles of gonadal steroid hormones in psychiatric depression in men and women. Prog Neuro-Psychopharmocol 1978;2:487–503
32. Rubin RT, Poland RE, Lesser IM. Neuroendocrine aspects of primary endogenous depression VIII: pituitary-gonadal axis activity in male patients and matched control subjects. Psychoneuroendocrinology 1979;14:217–229
33. Davies RH, Harris B, Thomas DR, Cook N, Read G, Riad-Fahmy D. Salivary testosterone levels and major depressive illness in men. Br J Psychiatry 1992;161:629–632
34. Barrett-Connor E, von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo study. J Clin Endocrinol Metab 1999; 84:573–577
35. Vogel W, Klaiber EL, Braverman DM. A comparison of the antidepressant effect of a synthetic androgen (mesterolone) and amitriptyline in depressed men. J Clin Psychiatry 1985;46:6–8
36. Morales AJ, Nolan JJ, Nelson JC, et al. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab 1994;78:1360–1367
37. Rabkin JG, Wagner GJ, Rabkin R. Testosterone therapy for human immunodeficiency virus-positive men with and without hypogonadism. J Clin Psychopharmacol 1999; 19:19–27
38. Rabkin JG, Wagner GJ, Rabkin R. A double-blind, placebo-controlled trial of testosterone therapy for HIV-positive men with hypogonadal symptoms. Arch Gen Psychiatry 2000; 57:141–147
39. Grinspoon S, Corcoran C, Stanley T, Baaj A, Basgoz N, Klibanski A. Effects of hypogonadism and testosterone administration on depression indices in HIV-infected men. J Clin Endocrinol Metab 2000;85:60–65
40. Rinieris PM, Malliaras DE, Batrinos ML, Stefanis CN. Testosterone treatment of depression in two patients with Klinefelter’s syndrome. Am J Psychiatry 1979; 136:986–988
41. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48:157–161
42. Seidman SN, Spatz E, Rizzo C, Roose SP. Testosterone replacement therapy for hypogonadal men with major depressive disorder: a randomized, placebo-controlled trial. J Clin Psychiatry 2001;62:406–412
43. Perry PJ, Yates WR, Williams RD, et al. Testosterone therapy in late-life major depression in males. J Clin Psychiatry 2002;63: 1096–1101
44. Pope HG, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105–111
45. Rolih CA, Ober KP. The neuroendocrine response to critical illness. Med Clin North Am 1995;79:211–224
46. Skarda ST, Burge MR. Prospective evaluation of risk factors for exercise-induced hypogonadism in male runners. West J Med 1998;169:9–12
47. Strollo F, Riondino G, Harris B, et al. The effect of microgravity on testicular androgen secretion. Aviat Space Environ Med 1998;69:133–136
< div class='tao-gold-member'>









