HIV-Associated Nephropathy
- HIV-associated nephropathy (HIVAN) is a distinct and separate pathology with a unique combination of collapsing focal segmental glomerulosclerosis (FSGS), tubuloreticular inclusion bodies, microcystic dilation of renal tubules, and interstitial inflammation found in HIV-infected individuals.
- While HIVAN should be suspected in any HIV-infected person of African descent with proteinuria and decreased glomerular filtration rate (GFR), definitive diagnosis can be made only by renal biopsy.
- At-risk HIV-positive patients should be tested for proteinuria or decreased GFR to allow earlier detection of HIVAN.
- HIVAN is caused by expression of HIV-1 genes in renal epithelial cells.
- Highly active antiretroviral therapy (HAART) is the primary mode of treatment to retard progression of glomerular filtration decline and proteinuria.
- The diagnosis of HIVAN is an indication to begin HAART regardless of CD4 or viral counts.
- Angiotensin-converting enzyme (ACE) inhibitors and steroids may be of benefit in selected patients.
At the onset of the HIV epidemic, little was known concerning the ability of the virus to cause organ-specific pathology. By 1984, it became apparent that there was a renal syndrome associated with HIV infection that was characterized by severe proteinuria and rapidly progressive renal failure. Proteinuria was frequently severe with levels of protein excretion as high as 10 g daily. Patients were often asymptomatic until features of renal failure became manifest. As opposed to similar glomerulopathies, the nephropathy associated with HIV infection did not often result in severe hypertension or edema. On ultrasound imaging of the kidneys, nephromegaly was a common finding with increased kidney weight seen on autopsy. Renal biopsy of these patients revealed the presence of focal glomerulosclerosis, tubular microcystic dilation, and tubulointerstitial inflammation and fibrosis.
Now widely accepted to be a distinct renal disease, HIVAN does not affect all populations equally. In the United States, HIVAN is the third leading cause of end-stage renal disease (ESRD) in African-Americans between the ages of 25 and 64 years. International studies evaluating HIV-infected patients with proteinuria corroborate the U.S. data. Two studies in Thailand and Italy did not detect any cases of HIVAN among HIV-infected patients who underwent renal biopsy to evaluate the cause of proteinuria. Neither of these studies included any patients of African descent. A South African study, however, evaluated 30 patients with proteinuria and 7 patients with microalbuminuria. Renal biopsies revealed that 83% of the patients had pathology consistent with HIVAN, including six out of the seven patients with microalbuminuria. Moreover, ESRD due to HIVAN is 12.2 times more likely to occur in African-Americans than in whites and has the strongest racial predisposition of any form of acquired renal disease leading to ESRD.
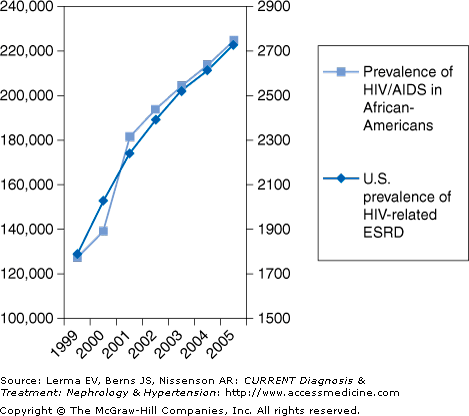
Prior to the availability of HAART, the clinical course of HIVAN was characterized by a rapid decline of renal function resulting in the need for renal replacement therapy within weeks to months after diagnosis. The prognosis of these patients was dismal, approximately 1 year once on dialysis. However, since HAART became widely available, the outcome of HIV-infected patients has improved significantly. Similarly, the prognosis of HIV-infected patients with ESRD has improved. After the introduction of HAART in 1995, the mortality rate fell from about 72% to 24% for patients with both HIV and ESRD. Unfortunately, despite the improvements in the efficacy of medical treatment, the prevalence of ESRD in persons living with HIV/AIDS has continued to increase. This increase is closely paralleled by the increased prevalence of HIV/AIDS in African-Americans, the group at highest risk of developing HIVAN (Figure 35–1).
Figure 35–1.
The prevalence of patients with end-stage renal disease due to AIDS nephropathy in the United States continues to increase in parallel with the prevalence of HIV/AIDS in African-Americans, the group at highest risk of developing HIV-associated nephropathy (HIVAN). (Data adapted from the U.S. Renal Data System, USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2007 and from the Centers for Disease Control and Prevention: HIV/AIDS Surveillance Report, 2005. Vol. 17, Rev ed. Atlanta, GA. Department of Health and Human Services, Centers for Disease Control and Prevention, 2007. Also available at http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Note: The data here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. Government.
Patients with biopsy-proven HIVAN most often present with obvious signs of renal involvement. Severe proteinuria and impaired GFR are common indications for biopsy in patients with renal disease. However, even the presence of microalbuminuria may herald the presence of HIVAN. In recent years, as HAART has become widely used, the classic clinical presentation of HIVAN has become less common. However, the incidence of ESRD due to HIVAN has not changed appreciably in the past 10 years. While there are no large recent biopsy series of patients with renal disease while on HAART, it is likely that HAART usage has transformed HIVAN from a rapidly progressive disease with severe proteinuria into an indolent disease with lower levels of proteinuria.
Acute kidney injury (AKI) and chronic kidney disease (CKD) are common problems in patients living with HIV/AIDS. Both outpatient and inpatient populations have been observed to have increased rates of AKI that are correlated with higher viral loads and lower CD4 counts; the most important predisposing factor to AKI is the presence of CKD. Interestingly, black race is not associated with increased risk for AKI in patients with HIV/AIDS, suggesting that HIVAN is not the underlying cause of CKD in most cases of AKI in HIV-infected patients. Analysis of over 2 million patients in the Veterans Administration (VA) database for a median time period of 3.7 years yielded a risk of ESRD for black individuals with HIV nearly 10 times that of white individuals with HIV. The most common cause of ESRD in blacks in this study was AIDS nephropathy, the diagnosis that is used by the U.S. Renal Data Service (USRDS) as a surrogate for HIVAN. Strikingly, HIV infection did not increase the risk of ESRD in white patients but did confer an increased risk of ESRD in black patients that was similar to diabetes. A similar study of VA patients with stage III or higher CKD again found a similar risk for ESRD and rate of GFR decline in black patients with HIV as compared to their counterparts with diabetes.
While ESRD is clearly an important problem in patients with HIV/AIDS, the incidence and prevalence figures from the USRDS do not account for persons living with CKD who have not yet reached ESRD. Previous studies have shown that the prevalence of HIVAN in African-Americans with HIV/AIDS is approximately 4–12%, a figure that has not changed appreciably during the past decade. Extrapolating this prevalence to the 224,815 African-Americans known to be living with HIV/AIDS living in the United States, we predict that there are currently 8992–26,977 African-Americans living with HIVAN in the United States. Moreover, 24.7 million of the 39.5 million people living with HIV/AIDS worldwide reside in Sub-Saharan Africa. Recent evidence suggests that the burden of HIV-related renal disease in Africans is similar to that reported in African-Americans. Applying the same prevalence figure leads us to predict that 0.98–3.0 million people currently have HIVAN in Africa. Since most Africans with HIV/AIDS currently receive little or no treatment for their illness, it is likely that many die of opportunistic infections before renal disease becomes clinically apparent. As the health care delivery to Africans with HIV/AIDS improves, particularly with expanding access to antiretroviral therapy (ART), increased survival of patients can be expected. Almost certainly, HIVAN will emerge as a major complication of HIV-1 infection and will become an increasing cause of morbidity and mortality in Africa.
The mechanisms through which infection with HIV-1 results in the HIVAN phenotype are not completely known. However, much research has been done to elucidate important aspects of disease pathogenesis mainly through the use of animal models that develop a phenotype analogous to HIVAN.
Initially, controversy existed as to whether HIVAN resulted from viral infection of the renal parenchyma or from the cytokine milieu created by infection and activation of lymphocytes throughout the body. An HIV-transgenic mouse model was created in which the mice express an HIV-1 provirus under control of the endogenous HIV viral promoter with deletions in the gag and pol genes, rendering the virus noninfectious. A subsequent experiment performed with this transgenic mouse involved reciprocal transplantation with a mouse not possessing the HIV transgene. Wild-type mice receiving a kidney with an HIV transgene developed proteinuria, glomerular, and tubular changes similar to HIVAN. HIV-transgenic mice, receiving a wild-type kidney, did not develop the diseased status. These data suggest that expression of HIV gene products within the kidney is necessary for the development of HIVAN.
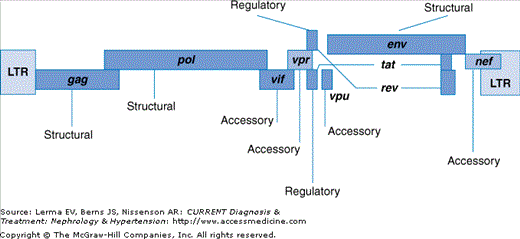
The HIV-1 genome is a 9 kb RNA that encodes nine genes, including three structural genes (env, gag, and pol) two regulatory genes (tat and rev), and four accessory genes (vif, vpr, vpu, and nef) (Figure 35–2). After processing, these nine genes yield 15 respective proteins, each of which plays a role in the life cycle of the virus. Of the 15 proteins, not all have been shown to be involved in the pathogenesis of HIVAN (Figure 35–3). The gag and pol genes (accounting for seven HIV proteins after proteolytic cleavage), for example, are not functional in the Tg26 mouse model of HIVAN, suggesting that their presence is not necessary for the development of disease. Further research has indicated two genes whose function is most important for the development of the HIVAN phenotype: nef and vpr.
Figure 35–2.
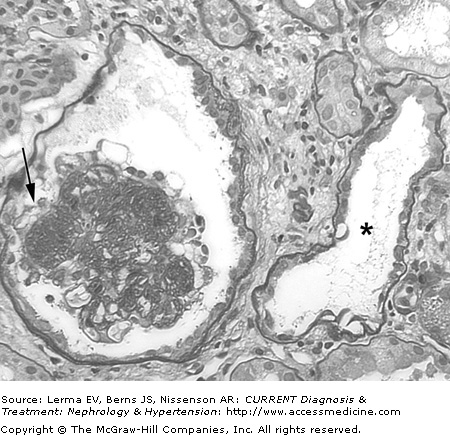
Typical histopathologic findings in HIV-associated nephropathy (HIVAN). Periodic acid–Schiff staining demonstrates focal glomerulosclerosis with collapse of the glomerular tuft and overlying podocyte proliferation (arrow), microcystic tubular dilation (asterisk), interstitial inflammation, and interstitial fibrosis (×200).
Several studies have attempted to determine the HIV-1 genes that are responsible for inducing HIVAN. In vitro studies have demonstrated that the nef gene is necessary and sufficient to induce the podocyte proliferation and dedifferentiation that are characteristic of HIVAN. Several transgenic murine models have also been developed to further delineate which viral genes are pathogenic to the kidney. HIV-transgenic mice that lack gag, pol, and nef develop FSGS. However, the breeding of these mice with nef-transgenic mice worsened the renal phenotype as compared to nef-mutant mice, suggesting that nef has deleterious effects in the kidney when other HIV gene(s) are present. In addition, transgenic mice expressing nef selectively in expression podocytes expression have increased podocyte proliferation and dedifferentiation, which are characteristic of HIVAN. However, these mice do not develop proteinuria or histologic glomerular disease, providing further evidence that while podocyte expression of nef is an important component of HIVAN pathogenesis, other HIV genes are necessary to induce the full phenotype.
Vpr has been demonstrated to have a number of pathogenic effects upon infected cells. These include induction of cell cycle arrest in the G2 phase, nuclear import of the HIV preintegration complex, transactivation of the viral long terminal repeat (LTR) promoter, and apoptosis. The ability of vpr to induce apoptosis has been linked to its ability to increase mitochondrial permeability. In vitro studies have demonstrated the rapid release of apoptogenic proteins (cytochrome-c and apoptosis-inducing factor) from mitochondria when exposed to a carboxy-terminal construct of vpr. Vpr also promotes apoptosis by selectively increasing expression of proapoptotic molecules including caspase-9. Studies using a series of transgenic mice expressing HIV genes demonstrated that deletion of vpr eliminated the renal disease phenotype, further supporting the critical role of vpr in HIVAN pathogenesis.
Though several published in vitro studies have suggested that expression of HIV-1 genes other than nef and vpr may affect renal cells, in vivo animal studies have not supported a role for these genes in HIVAN pathogenesis. Several transgenic lines were created, each expressing one HIV gene (vif, vpr, vpu, nef, rev, tat) except for gag and pol, under the control of the podocyte-specific Nphs1 promoter. Expression of the HIV transgene in the kidney was confirmed by real time polymerase chain reaction (PCR). No mouse expressing the transgenic product of rev, tat, vif, or vpu developed proteinuria or histologic glomerular lesions. Supporting the findings of previous studies, only transgenic mice expressing nef and/or vpr developed proteinuria and glomerular abnormalities, including FSGS. Furthermore, double-transgenic mice with kidney-specific expression of both vpr and nef developed proteinuria 1 week, as compared to 4 weeks, in mice transgenic for vpr or nef alone. Nef–vpr transgenic mice also developed markedly worsened renal function as compared to vpr transgenic and nef single transgenic mice by 4 weeks.
Taken together, the preponderance of published studies support the role of HIV-1 vpr and nef as the most important viral genes in the pathogenesis of HIVAN. While expression of either gene in the kidney can reproduce stereotypical features of HIVAN histology, coexpression results in the most severe phenotype, indicating an additive or synergistic effect of these genes.
The most common pathologic finding in HIVAN biopsy specimens is focal glomerulosclerosis (FGS). This FSGS is often associated with retraction of the glomerular capillary walls resulting in narrowing of the lumen and wrinkling of the glomerular basement membrane causing the glomerulus to appear collapsed and is therefore described as “collapsing focal glomerulosclerosis.”
The visceral epithelial cells of the glomerulus, known as podocytes, undergo characteristic phenotypic changes in HIVAN. Podocytes are usually terminally differentiated, quiescent cells, however, in HIVAN, they often become hypertrophic and proliferate. Prominent tubulointerstitial disease is present, the severity of which may exceed the glomerular pathology. Interstitial infiltrates are pleomorphic and may consist of lymphocytes, plasma cells, and macrophages. The tubular abnormalities include flattening and atrophy of tubular epithelial cells and dilation of the tubular lumen, which can be filled with proteinaceous casts. This “microcystic” dilation of the tubules is characteristic of HIVAN and can take place among multiple segments of the nephron.
On electron microscopy, several findings characterize the HIVAN lesion. There is almost complete effacement of podocyte foot processes and the visceral epithelial cytoplasm can have large, electron-dense resorption droplets. The endothelium has large tubuloreticular inclusions located within the cisternae of the endoplasmic reticulum or the Golgi bodies. This can also be seen in patients with lupus nephritis. However, is often not present in patients with the idiopathic collapsing variant of FSGS and therefore should alert the clinician to the possibility of HIVAN if the patient is HIV positive or if the HIV status of the patient is unknown.
Strategies for the prevention and/or treatment of HIVAN have never been evaluated in prospective randomized controlled studies and most studies on the treatment of HIVAN have been retrospective and/or lack proper controls. Three types of medical therapy for HIVAN have been studied in humans: Antiretroviral therapy, corticosteroids, and ACE inhibitors.
Studies demonstrating that HIV infection of kidney parenchymal cells is necessary for the development of HIVAN support the hypothesis that antiretroviral medications should be beneficial for treating and/or preventing HIVAN. Early papers on the use of antiretroviral therapies were mainly case reports involving zidovudine. The first of multiple reports of HIVAN regression in response to zidovudine therapy was published in 1989. A retrospective study from France of patients with biopsy-proven HIVAN found that past use of antiretroviral therapy was associated with a relative risk of 1.9 for progression to ESRD. It was not clear whether use of antiretroviral medications worsened the outcome or if patients who progressed while on therapy were a treatment-resistant group and therefore at higher risk of worsening renal function.
Two of the largest studies looking at the effect of antiretroviral therapy in patients with HIVAN were both retrospective. The first study, published in 2004, assessed 3976 patients in a Baltimore AIDS clinic. Over the 12-year course of the study, HIVAN was diagnosed by biopsy or a conservative clinical protocol in 94 of the patients. Patients with HIVAN were evaluated in groups based on their treatment. The incidence of HIVAN was found to be 26.4, 14.4, and 6.8 per 100 patient-years in groups receiving no treatment, treatment with nucleoside analog only, or treatment with HAART, respectively. In addition, the study demonstrated a significant decrease in the incidence of HIVAN in the years 1998–2001 as compared to 1995–1997. The use of HAART appeared to be superior to nucleoside analog treatment alone, and appeared to account for the decreased incidence of HIVAN diagnosis. The decreasing incidence of HIVAN in this study contrasts with the relatively unchanged incidence of ESRD due to HIVAN over the past decade. This may reflect a change in the HIVAN phenotype in patients treated with HAART resulting in a more indolent disease that is less likely to be evaluated by diagnostic renal biopsy.
The second study, published in 2006, involved a cohort of 263 consecutive HIV-infected patients referred to the renal clinic at Johns Hopkins University. Of the 263 patients, 53 had biopsy-documented HIVAN. Seventeen were excluded because they required dialysis within a month of diagnosis. The characteristics of the remaining 36 were evaluated and compared with regard to their outcomes. Multivariate analysis demonstrated a significantly lower progression of disease with ART (hazard ratio of 0.30). The median renal survival was found to be 552 days in the treated group and 117 in the untreated group. Since this study included patients treated with nucleoside analog solo therapy (prior to the availability of HAART) and those treated with HAART, these results may underestimate the effect of modern ART upon renal outcomes.
Unfortunately, there have been no randomized controlled trials to evaluate the use of HAART in treating HIVAN. Since most patients with HIVAN have other indications for treatment with HAART, randomized placebo-controlled trials are not ethically feasible. Fortunately, the published literature continues to support the use of HAART in patients with HIVAN. As a result, the Infectious Diseases Society of America guidelines now state that a diagnosis of HIVAN is a strong indication for initiating HAART regardless of viral load or CD4 count.
The effect of stopping ART in patients previously diagnosed with HIVAN has not been studied. However, a case report of a woman who developed recurrent HIVAN after stopping HAART suggests that cessation of treatment may not be safe once a diagnosis of HIVAN is made and if antiretroviral medications must be withheld, patients should be monitored for worsening renal function or proteinuria.
Decades have passed since zidovudine was the sole antiretroviral available to treat chronic HIV infection. Available antiretroviral medications are increasingly diverse in their mechanisms of action and include nucleoside and nonnucleoside reverse transcriptase inhibitors, protease inhibitors, integrase inhibitors, and viral entry inhibitors. The ability to control viral replication and enact immune reconstitution therefore continues to improve. Hopefully, these advances in the treatment of HIV/AIDS will eventually translate into a decrease in the morbidity and mortality from HIV-mediated renal disease in these patients.
Blockade of the renin–angiotensin system (RAS) has been shown to attenuate proteinuria in various glomerular diseases. There are also data from studies in HIV-transgenic models of HIVAN that suggest that RAS blockade using ACE inhibitors or angiotensin receptor blockers can decrease proteinuria and improve histologic and functional renal outcomes.
ACE inhibitors have been evaluated in several small studies. Initially, case reports in the early 1990s supported a beneficial role of ACE inhibitors in decreasing proteinuria and preserving renal function in HIVAN, but conclusions regarding the effect of these medications were made difficult by the concomitant use of ART. In 1996, a retrospective case–control study found that use of captopril was associated with slower progression to ESRD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree