1. Cholestatic disease
(a) EHBA
33.6 %
(b) Alagille and nonsyndromic paucity
4.6 %
(c) Sclerosing cholangitis
1.3 %
(d) Familial cholestasis
2.5 %
(e) Choledochal cyst
0.3 %
(f) Others
8.4 %
2. Metabolic liver disease
(a) Alpha-1-antitrypsin deficiency
3.5 %
(b) Glycogen storage II/IV
0.9 %
(c) Tyrosinemia
1.1 %
(d) Primary oxalosis/oxaluria
0.9 %
(e) MSUD
3.8 %
(f) Wilson’s/other copper defects
1.5 %
(g) Hemochromatosis
0.1 %
(h) Others
2.0 %
3. Fulminant hepatic failure
(a) Unknown
3.7 %
(b) Drugs
1.2 %
(c) Others – viral
1.2 %
4. Tumors
(a) Hepatoblastomas
3.4 %
(b) HCC
1.0 %
(c) Others
0.6 %
5. Congenital anomalies
(a) Cystic liver disease
0.6 %
(b) Congenital hepatic fibrosis
2.3 %
6. Neonatal hepatitis
1.9 %
7. Cirrhosis
(a) TPN injury as part of multivisceral
11.7 %
(b) Viral hepatitis
0.6 %
(c) Autoimmune
0.7 %
(d) Cryptogenic
2.8 %
(e) Others
0.5 %
8. Miscellaneous
(a) Cystic fibrosis
2.1 %
(b) Budd-Chiari
0.7 %
(c) GVHD
0.2 %
(d) Others
0.3 %
The surgical specimens in many cases require special handling, depending on the primary disease for which the transplant was done. They also serve as a useful reservoir for study of liver pathology in the pediatric age group (Fig. 15.1). In many situations fresh tissue may be needed to be frozen for special studies such as with mitochondrial diseases or metabolic conditions and tumors, as well as submitted for electron microscopy where indicated. Intraoperative evaluation of the resected specimen is rarely required, but may be required in cases of neoplastic disease where the surgeon wants to assess the adequacy of the surgical margins, especially in tumors that extend beyond the limits of the liver parenchyma.
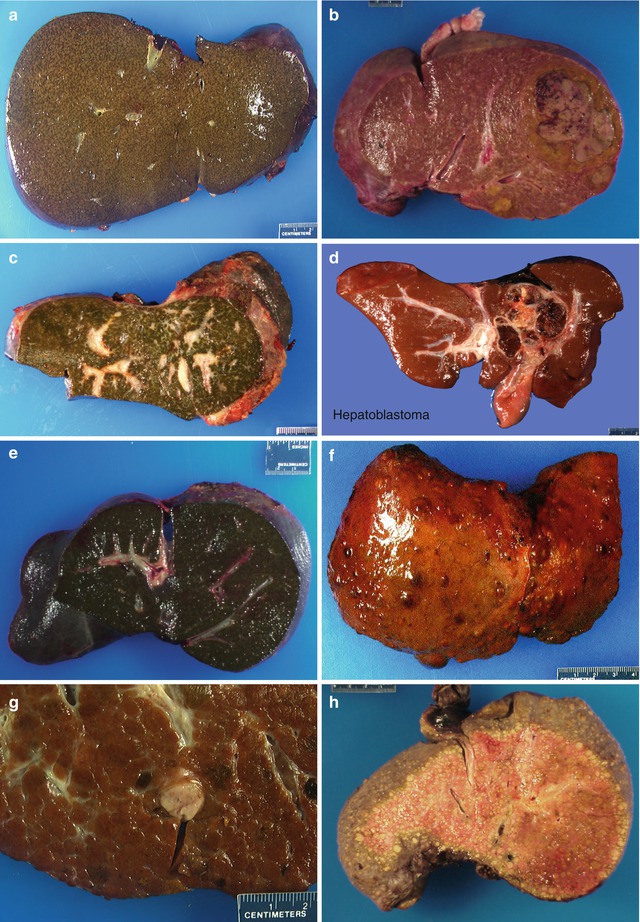

Fig. 15.1
Gross images of explant liver. (a) Case of Alagille syndrome. (b) Child with congenital hepatic fibrosis with an adenoma. (c) An example of extrahepatic biliary atresia post failed Kasai. (d) An example of hepatoblastoma with tumor involving the hilar region, post chemotherapy. (e) An example of cholestatic liver due to total parenteral nutrition. (f) A cirrhotic liver due to tyrosinemia. (g) Another liver of tyrosinemia patient with incidental HCC. (h) Cirrhotic liver in a child with Wilson’s disease
15.1.2 Role of the Pathologist
The pathologist plays a primary role in the diagnosis of native diseases, intraoperative assessment of donor tissue where indicated, and in the postoperative management of transplant complications (Lerut et al. 1988; Demetris et al. 1987). Other than assessment of surgical margins in cases of malignancy of the native liver, as mentioned above, frozen sections at the time of transplant procedure are usually done for donor organ evaluation, when the surgical team is not sure of the donor disease course prior to his/her demise in situations of cadaveric transplant (Kakizoe et al. 1990b). The main concern for intraoperative assessment of the donor liver is for steatosis and, while routinely performed in adult centers, this indication is much less frequent in the pediatric age group. There is data to show that steatosis in the donor liver, especially the microvesicular type, is associated with an adverse graft survival and increased complications, especially acute cellular rejection (Todo et al. 1989). Determination of the percentage of liver parenchyma involved by steatosis is the main role for the pathologist in this situation. Other findings such as evidence of hepatitis, portal inflammation, or even incidental cirrhosis due to undetected primary liver disease may alert the surgeon to not use the donor organ for transplant. In most instances, at least in pediatric transplants, the gross appearance of the liver is sufficient to decide on the suitability for transplant, and this decision is made by the surgeon.
Posttransplant handling of biopsy or tissue specimens from an allograft liver is a critical step for diagnosis of complications. Early posttransplantation, the biopsy can be entirely fixed in formalin if the suspicion is mainly rejection. As infections frequently occur in the first month posttransplant, or whenever there is a suspicion of infection, we recommend getting two cores, submitting one in formalin and the other fresh for microbiological cultures. Electron microscopy is very rarely needed in the early postoperative evaluation of liver allograft biopsies. In major transplant centers such as the Children’s Hospital of Pittsburgh, rapid processing of transplant biopsies is available as an option, wherein a biopsy done in the morning is processed on a two and a half to 3 h automated processing cycle and is available for a final read the same day. This facilitates management of patients in whom sudden variations in liver functions may require immediate changes in therapy. While routine hematoxylin and eosin (H&E) staining may be sufficient, it is helpful in many instances to do a PAS stain with diastase digestion, a trichrome stain, and possibly a reticulin stain to study the overall architecture of the liver biopsy. Immunohistochemical markers for bile duct proliferation, such as cytokeratin AE1/AE3 or cytokeratin 7, may be indicated in cases where biliary complications are suspected or in late biopsies for assessment of chronic rejection. While the AE1/AE3 stain is a good marker to study bile duct injury in rejection and in other complications, it does not highlight to the same extent the degree of ductular proliferation seen in biliary obstruction as the CK7 immunostain. Specific stains for infectious organisms may be performed where indicated, and these include bacterial and fungal stains in biopsies that show the presence of abscesses or neutrophil collections and viral stains such as cytomegalovirus (CMV), herpes, and adenovirus immunostains in suspected cases of opportunistic viral hepatitis. Epstein-Barr virus (EBV) in situ hybridization for EBV-encoded RNA (EBER-1) is done in cases where serology and/or EBV PCR turns positive or is high and when the histological pattern is that of hepatitis. It cannot be adequately stressed that allograft liver biopsies need to be interpreted in the light of liver function tests as well as in consultations with the transplant team to arrive at the right diagnosis. It is imperative to have at least an ALT, AST, GGTP, and bilirubin value in hand while evaluating the biopsies. Other significant data such as analyses for viral infections and levels of immunosuppressive agents also aid in the interpretation. Correlation with imaging studies will also help evaluate the biliary tree and vascular structures. Information on any immediate treatment given prior to the biopsy will also help assess the biopsy specimen. As a routine, every biopsy at CHP is reviewed with the clinician usually prior to sign-out so as to arrive at the right diagnosis.
15.1.3 Postoperative Complications
There are several factors that determine the occurrence of immediate postoperative complications following transplant, the type of donor organ, the time taken for organ retrieval and transport, the time to transplant, and in some instances the duration of vascular clamp time, all of which play a role in the postoperative function of the allograft. In our center, a baseline liver biopsy is frequently performed immediately postperfusion of the organ. The changes seen in this biopsy may be entirely nonspecific, showing some minor hemorrhages in the subcapsular region and some inflammatory cells, or may show more dramatic changes of parenchymal necrosis and steatosis (Fig. 15.2a). In rare instances, incidental donor diseases such as Von Meyenburg complexes that may have no clinical significance may be encountered (Fig. 15.2b, c). This baseline biopsy serves as a guide when comparing subsequent biopsies in the postoperative period for evaluation of complications.
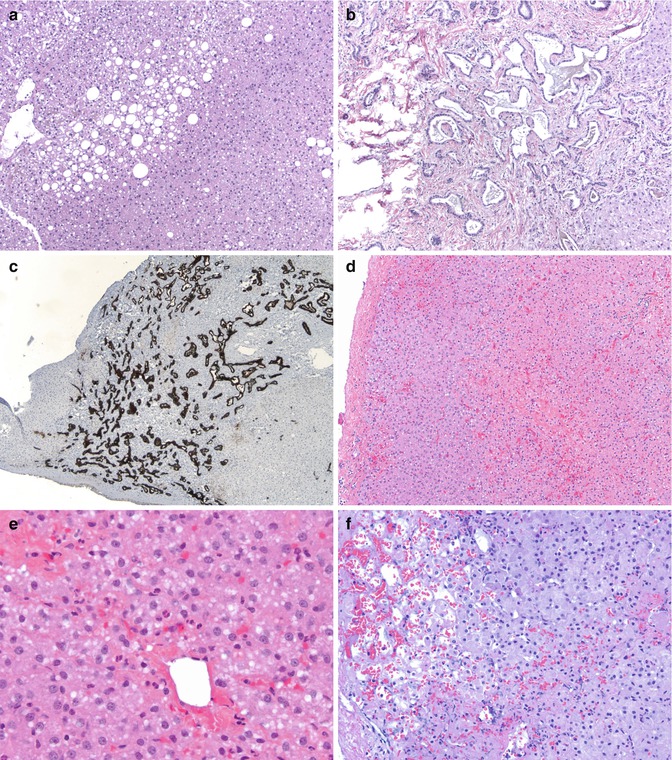

Fig. 15.2
Donor liver biopsy and reperfusion injury: (a) An image of a day 0 donor liver biopsy showing macrovesicular steatosis in about 30 % of parenchyma (HE ×100). (b) Photomicrograph of a donor liver biopsy showing presence of Von Meyenburg complexes (HE ×100). (c) A cytokeratin 7 stain highlighting the Von Meyenburg complex (×40). (d) Another day 0 biopsy showing centrilobular congestion and ballooning of hepatocytes with some loss of hepatocytes (HE ×100). (e) Higher magnification of same showing centrilobular ballooning, early steatosis, and some neutrophils (HE ×400). (f) Another day 0 biopsy post-reperfusion showing subcapsular sinusoidal dilatation with congestion and some centrilobular dropout of hepatocytes (HE ×200)
15.1.3.1 Primary Graft Nonfunction
Primary graft nonfunction is an uncommon complication in the pediatric age group, as it is frequently associated with preexisting fatty infiltration of the liver. It is thought to be due to ischemic injury, and histology reveals the presence of large coalescent globules of fat in the mid-zonal and centrilobular areas, along with sinusoidal congestion and hemorrhage and mild neutrophilic infiltration. It is not thought to be an immunologically mediated injury, such as hyperacute rejection or antibody-mediated rejection, but is likely due to a mechanical disruption of the hepatic sinusoidal microvasculature (Todo et al. 1989). This can be a life-threatening complication and requires removal of the graft and re-transplantation. In some instances, primary nonfunction has also been seen in the first month posttransplant (Kemmer et al. 2011).
15.1.3.2 Preservation Reperfusion Injury
This injury is usually the result of donor organ damage caused by terminal events in the donor in cases of cadaveric transplants or related to organ preservation causing cold ischemia and subsequent reperfusion of the organ (Kakizoe et al. 1990a). While cold ischemia and preservation fluid damage sinusoidal endothelial cells, reperfusion leads to the release of endotoxins and tumor necrosis factor (TNF), which activate Kupffer cells to release reactive oxygen species and cause liver damage (Bilzer and Gerbes 2000; Teoh and Farrell 2003). The biliary tree is often the target of preservation reperfusion injury. It is manifested by a significant increase of serum amino transferases ALT and AST in the first few days after transplantation, followed by persistent elevation of bilirubin and gamma-glutamyl transpeptidase (GGTP) (Rosen et al. 1998).
15.1.3.2.1 Pathology (Fig. 15.2d–f)
Biopsy findings show variable changes depending on the severity of the injury. While mild injury manifests as microvesicular steatosis and hepatocellular swelling, more severe injury is characterized by confluent necrosis, as well as neutrophilic infiltration. Canalicular cholestasis is also seen in the centrilobular areas and can persist for days. There may be evidence of liver regeneration with mitoses and rounding up of hepatocytes a few days post injury. Ductular injury is not seen, but there may be evidence of neutrophilic pericholangitis. Severe injury with periportal confluent necrosis can lead to ductular proliferation, and the severity of ischemic injury may result in the development of biliary obstruction with stricture formation as a final consequence. Complete recovery is, however, possible with restoration of normal architecture and function in most cases of preservation injury (Demetris et al. 1987).
15.1.3.2.2 Differential Diagnosis
Antibody-mediated rejection, biliary obstruction due to surgical reasons, sepsis, and acute cellular rejection (ACR) can produce similar changes posttransplant. Correlation with predisposing donor organ factors such as those listed above or with donor circulating antibodies and C4d stain or imaging studies to examine the biliary tree may be warranted to exclude the aforementioned causes. Absence of lymphocyte-mediated direct biliary injury and of a mixed portal infiltrate differentiates ACR from reperfusion injury. Acute cellular rejection may sometimes be superimposed and needs to be recognized for appropriate treatment.
15.1.3.3 Small-for-Size Syndrome (SFSS) (Fig. 15.3)
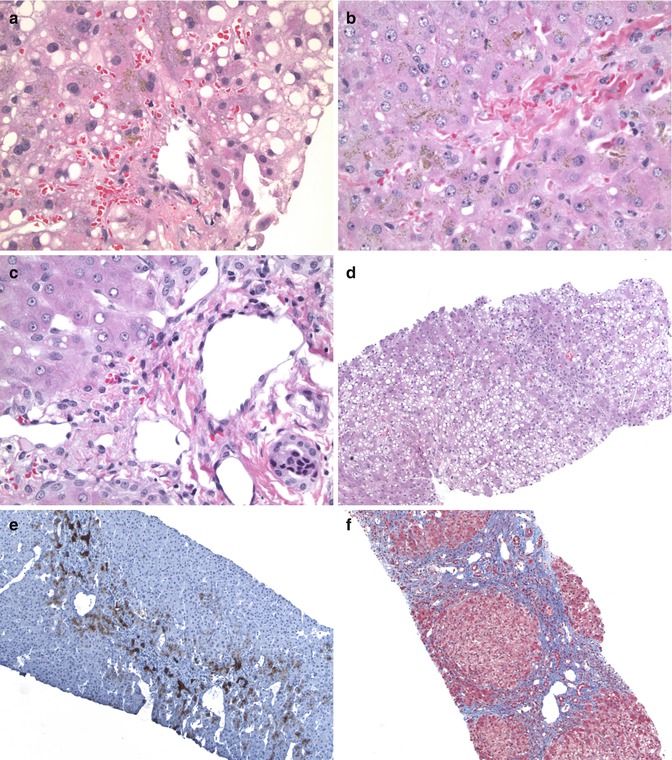
Fig. 15.3
An example of small-for-size liver transplant: (a) The centrilobular area shows macrovesicular steatosis with some perivenular extravasation of red cells (HE ×400). (b) Another lobule showing prominent canalicular and intracellular cholestasis (HE ×400). (c) A portal area showing dilated collaterals and venules surrounding the portal vein (HE ×400). (d) A biopsy after a few weeks showing extensive macrovesicular steatosis (HE ×100). (e) A cytokeratin 7 stain showing the prominent bile ductular reaction at the edges of the portal area (CK7 DAB ×100). (f) Same patient’s liver biopsy 1 year later showing bridging fibrosis and regenerative nodule formation (Masson trichrome ×100)
This is now recognized as a complication in living donor or reduced-sized liver transplants. By definition, the transplanted donor segment is less than 30 % of the standard or expected liver volume of the recipient or less than 0.8 % recipient body weight (Lo et al. 1996a, b; Troisi et al. 2005). SFSS is characterized by postoperative coagulopathy and liver dysfunction due to insufficient functional liver mass. It is manifested as portal hypertension with progressive cholestasis and ascites (Tucker and Heaton 2005). While most cases have been seen in adults, we have encountered occasional cases in children (Demetris et al. 2006b). It is thought to be due to a disruption in the balance between portal venous and hepatic artery inflow with sudden portal hyperperfusion following anastomosis causing hepatic arterial vessel spasm. This leads to decreased hepatic arterial flow and ischemic injury that manifests as centrilobular steatosis and ballooning, as well as infarcts. The hyperperfusion also causes portal venous endothelial injury with resultant hemorrhage into perivenous tissue and into the lobules. Over time, this can lead to portal venous thrombosis and associated ischemic injury with cholestasis, ductular reaction, and progressive cirrhosis with nodule formation (Fig. 15.3f). Early recognition of this condition with surgical intervention to decrease portal venous inflow helps in the prevention of the small-for-size syndrome (Beath et al. 1993).
15.1.3.4 Hepatic Artery Thrombosis (Fig. 15.4)
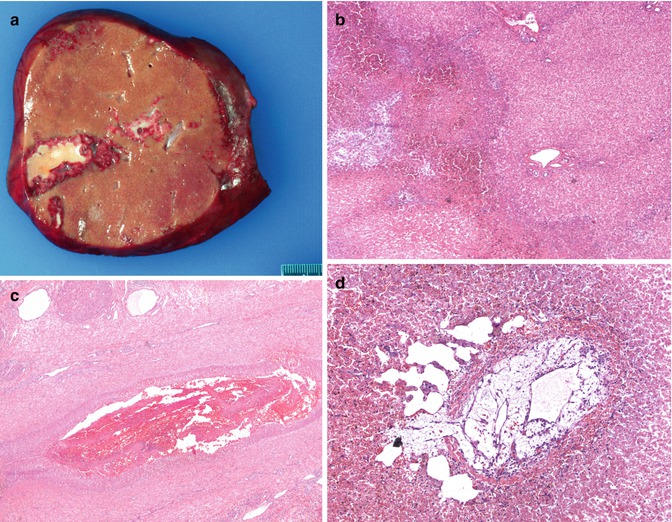
Fig. 15.4
Hepatic artery thrombosis: (a) Gross image showing a wedge-shaped infarct. (b) Low power photograph showing an area of ischemic parenchymal damage (HE ×400). (c) Hilum of the liver from a resected allograft showing early fibrin thrombus in a hepatic artery (HE ×40). (d) A central vein in the same case showing an organizing thrombus with recanalization. Note the dilated sinusoids suggesting outflow obstruction (HE ×100)
The hepatic artery primarily supplies the extra- and intrahepatic bile ducts and the hilar and portal tract connective tissue. Arterial thrombosis is a major cause of early allograft dysfunction and failure and usually occurs within the first few postoperative weeks (Mor et al. 1993). Mazariegos et al. reported an incidence of 7.5 % in their series of 416 OLT in 375 consecutive pediatric patients (Mazariegos et al. 1999). Children appear especially predisposed to ischemic liver injury since they have smaller caliber vessels, and hence more difficult anastomotic procedures (Heffron et al. 2003; Marangoni et al. 2008; Mazzaferro et al. 1989; Rela et al. 1996). The lack of collaterals also perpetuates this injury (Tan et al. 1988). Patients may be asymptomatic or can present with biliary symptoms such as pain, fevers, and evidence of cholangitis. Large areas of necrosis can cause fulminant liver failure. Early recognition may lead to attempts at hyperbaric oxygen therapy or surgical removal of the thrombus and prevention of significant damage (Mazariegos et al. 1999). Jurim et al. suggested that the incidence of HAT following split liver grafts was much lower than for pediatric whole liver transplants with an incidence of 0 versus 29 % in their cases (Jurim et al. 1995).
15.1.3.4.1 Pathology
A needle biopsy may not be the optimal way to make a diagnosis of hepatic artery thrombosis due to the sampling error involved. When the changes are extensive, a biopsy may reveal frank coagulative necrosis or centrilobular hepatocyte swelling and subsequent cholangiolar proliferation with or without bile plugs. An acute cholangitis may also develop, and the end result may be biliary strictures and obstruction. Secondary infection of necrotic areas may result in sepsis. Hepatic artery thrombosis can result in the need for re-transplantation if changes are advanced (Abou El-Ella et al. 2001; Stringer et al. 2001).
15.1.3.5 Portal Vein Thrombosis (Fig. 15.5)
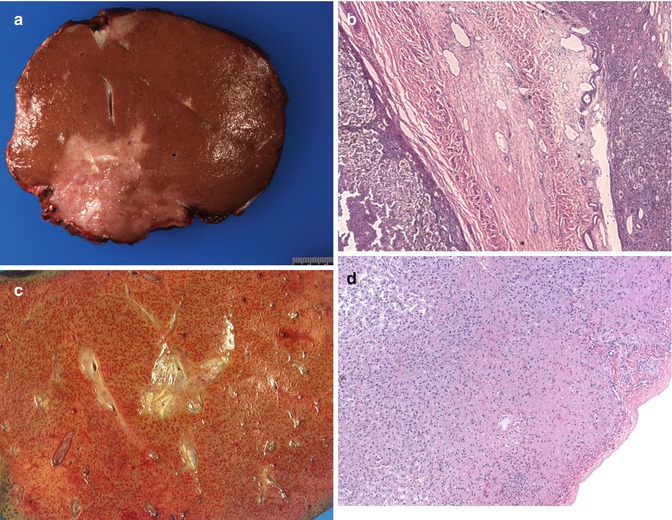
Fig. 15.5
Early complications post liver transplant (Tx): (a) Gross image of resected allograft showing an irregular area of infarction. (b) A large portal vein radical showing complete obliteration of the lumen by an organized thrombus (HE ×40). (c) A graft resection showing a mottled liver with congestion in a child with primary nonfunction. (d) A postperfusion biopsy showing a zone of subcapsular parenchymal necrosis (HE ×100)
Portal vein thrombosis (PVT) is less common than arterial thrombosis but has been reported in the pediatric age group (Millis et al. 1996; Corno et al. 2005). Small PV size (<4 mm) and slow portal flow (<10 cm/sec) combined with lesser hepatic artery resistance index (<0.65) are strong warning signs that may predict the development of post-living donor liver transplant (LDLT) PVT in EHBA patients requiring close monitoring (Ou et al. 2011). It can result in fulminant liver failure or ascites. Needle biopsies may not be helpful in making the diagnosis (in fact may miss the diagnosis), and radiological studies may be more helpful (Millis et al. 1996).
15.1.3.6 Subcapsular Hepatic Necrosis and Hemorrhage (Fig. 15.5d)
This is a pattern of hepatic infarction that has been noted in pediatric allografts, the exact etiology of which is not clear (Russo and Yunis 1986). Vascular thrombosis has not been identified in these patients, and it is thought to occur as a result of hypotension and shock. This can sometimes be seen as an incidental finding and may not influence the functioning of the allograft. Specific cases can lead to cardiac failure and warrant removal of the allograft. Histologically, this is manifested as areas of necrosis with extensive ductular proliferation and primarily neutrophilic portal inflammatory cell infiltrates in a subcapsular zone. Frank hemorrhagic infarction may also be seen in this area.
15.1.4 Rejection
Allograft rejection has been defined as “an immunological reaction to the presence of a foreign tissue or organ which has the potential to result in graft dysfunction and failure” (Demetris et al. 1997a). There are several mechanisms by which a recipient can reject the donor allograft. The main reason is usually a genetic disparity between the donor and recipient and may be related to either blood group or major histocompatibility antigens (MHC antigens). Sindhi et al. have shown that the HLA-DOA gene and the B lymphocyte, in which it is exclusively expressed, are plausible candidates contributing to pediatric liver transplant rejection (Sindhi et al. 2008). The exact mechanism, however, is unclear. Starzl et al. have shown that tolerance is frequently observed in long-term liver allograft survivors (Starzl 2004; Starzl et al. 1992). They have also shown that as tolerance of the graft develops, recipients progressively require less maintenance immunosuppression and can sometimes be completely withdrawn off medications (Starzl and Zinkernagel 2001). It has also been shown that recipients of living-related donors can be more successfully weaned than mismatched cadaveric donors.
Rejection can be divided into antibody-mediated rejection, acute cellular rejection (ACR), and chronic rejection. Antibody-mediated rejection usually occurs within the first few weeks of transplantation, while ACR can occur at any time posttransplant. Chronic rejection is usually the result of persistent or progressive acute cellular rejection and may develop in months to years.
15.1.4.1 Antibody-Mediated Rejection (AMR) or Humoral Rejection
Liver allografts in general are resistant to hyperacute or AMR from preformed lymphocytotoxic antibodies. This is thought to be due to the dual liver blood supply as well as the ability of the Kupffer cells to remove deposited immune complexes. Despite this, ABO incompatibility as well as preformed antibodies do lead to manifestations of hyperacute rejection and can present immediately posttransplant. The mechanism of AMR is thought to be due to endothelial injury caused by binding of antibodies to ABO and HLA antigens located on the sinusoidal endothelial cells. This results in endothelial damage with platelet thrombi formation, initiation of the clotting cascade, intravascular thrombosis, and impaired blood supply leading to hemorrhagic necrosis (Demetris et al. 1988, 1992b; Starzl et al. 1989; Manez et al. 1993). This is sometimes seen at the time of surgery as a cyanotic mottled flaccid liver that fails to produce bile and may warrant removal of the graft. In other instances, AMR presents with early graft dysfunction and can result in progression to chronic rejection with loss of bile ducts over a period of time. More recent studies have demonstrated donor-specific HLA antibodies (DSA) in rejection and resulting ductopenia even in ABO compatible liver transplantation. It has been demonstrated that DSA as well as C4d staining may be seen in livers with associated ACR and that coexistence of AMR and ACR may play a more significant role in progression to ductopenic rejection (Musat et al. 2011; Sakashita et al. 2007). The diagnosis is made when all of the following criteria are met: early graft failure, light and immunofluorescent findings, demonstration of a presensitized state in the recipient, and demonstration of circulating donor-specific antibodies. Progressive increase of AST, ALT, and GGTP may be indicators of AMR. A persistent rise of bilirubin and thrombocytopenia may also be seen.
15.1.4.1.1 Pathology
The biopsy manifestations of AMR are dependent on the timing of the biopsy and the type of the circulating antibody. The manifestations include sinusoidal congestion, neutrophilic sludging, and platelet fibrin thrombi in portal and central vessels. Single cell hepatocyte necrosis as well as confluent necrosis in severe cases may be seen. Necrotizing arteritis may also be seen in severe cases and progressive hepatic infarction can result (Demetris et al. 1988). The major consequences of AMR include biliary sludging, bile duct strictures, obliterative arteriopathy, progressive bile duct loss, and chronic rejection. Immunohistochemical staining with C4d may be useful, especially if there is diffuse staining along sinusoidal endothelial cells as well as in the portal veins and arteries. It is important to remember that C4d deposition may also be seen in cases of acute cellular rejection in the absence of well-documented AMR. It is interesting to note that Ali et al. observed C4d deposition mainly in the portal veins, capillaries, and arteries in their two cases of possible AMR and only faint to no staining in the sinusoids (Ali et al. 2012). Correlation with circulating donor-specific lymphocytotoxic antibodies or ABO antibodies is necessary to make a definitive diagnosis of AMR. We have encountered occasional cases wherein the morphology raised the question of coexisting AMR with ACR, but there was no consistent correlation between DSA and C4d staining in these cases.
The differential diagnosis of AMR includes preservation reperfusion injury which can be distinguished by demonstrating circulating donor-specific antibodies as well as by diffuse C4d staining. Portal infiltrates are not a feature of AMR and should raise the possibility of coexisting disease, either biliary complications or ACR. In many situations, ACR frequently coexists with AMR. It is also significant that studies of C4d staining in pediatric inflammatory liver diseases have shown deposition in 83 % of autoimmune hepatitis biopsies, 40 % of hepatitis C biopsies, and 89 % of hepatitis B biopsies. The staining was noted to be present in sinusoids as well as portal and central veins (Bouron-Dal Soglio et al. 2008).
15.1.4.2 Acute Cellular Rejection
ACR is defined by inflammation of the allograft that involves both portal elements and the hepatic veins with secondary injury to the lobules. It is usually recognized between 5 and 30 days posttransplant but can be seen as early as 3 days and can also manifest much later in the life of an allograft. Immediately posttransplant there is an exchange and migration of donor and recipient lymphocytes resulting in sensitization of the host and production of cytotoxic lymphocytes, with interaction between recipient lymphocytes and donor dendritic cells causing T-cell activation. This results in cytokine release and proliferation of cytotoxic lymphocytes with formation of immature blastic forms and migration of other inflammatory cells into the graft. Due to the localization of the graft dendritic cells in portal areas, the latter appear to be the main site of inflammatory infiltration and injury. The centrilobular damage is likely a result of circulating anti-donor antibodies in addition to cytokine injury to sinusoidal endothelium (Starzl et al. 1989; Demetris 1992; Demetris et al. 1991).
Patients may have fever with nausea and vomiting, as well as tenderness of the graft. Liver dysfunction manifests as three- to fourfold elevation of standard liver enzymes, including AST, ALT, GGTP, and alkaline phosphatase, as well as elevations in serum bilirubin. Peripheral leukocytosis and eosinophilia may also be seen, but these are nonspecific findings. Since there is an overlap in the laboratory parameters with other etiologies, a liver biopsy is usually warranted for the diagnosis of ACR.
15.1.4.2.1 Pathology (Fig. 15.6)
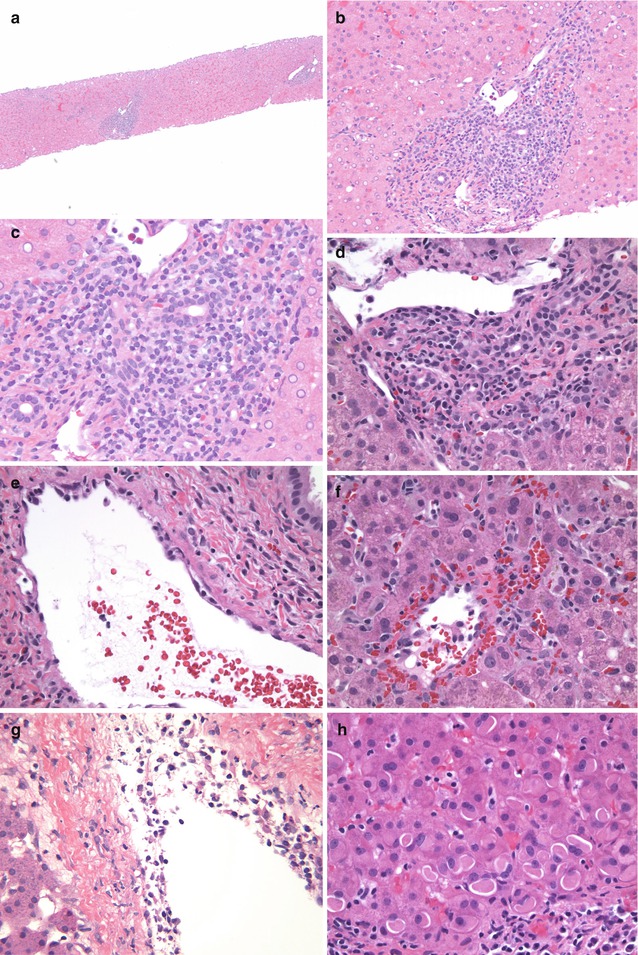
Fig. 15.6
ACR – liver Tx: (a) A scanning image of a liver biopsy showing expanded portal areas with inflammatory infiltrate (HE ×40). (b) Higher magnification highlighting the portal infiltrate composed of lymphocytes, activated “blastic” lymphocytes, and eosinophils. Note the bile ductular injury with crowding and overlap of nuclei. There is no interface activity (HE ×100). (c) High magnification image showing the biliary injury and intraepithelial lymphocytes (HE ×400). (d) Another example showing a more prominent eosinophilic component in the infiltrate with biliary damage and portal vein endotheliitis (HE ×400). (e) Portal vein showing lifting up of endothelial cells by lymphocytes – endotheliitis in ACR (HE ×400). (f) Central perivenulitis highlighted by extravasation of erythrocytes with CV endotheliitis and extension of lymphocytes into the wall of the vein (HE ×400). (g) Another example depicting the prominent central perivenulitis with lymphocytes within the intima and wall of the CV (HE ×200). (h) Biopsy showing prominent cytoplasmic eosinophilic globular inclusions (polyglucosan inclusions) (HE ×400)
Pediatric ACR can manifest as predominantly portal or centrilobular changes, or a combination of both (Starzl and Demetris 1990a, b; Kakizoe et al. 1990b; Demetris et al. 1987; Jaffe and Yunis 1989). An adequate needle biopsy would be one that includes about eight to ten portal areas as well as a similar number of central veins within the core (Demetris and Ruppert 2003).
Portal Changes: The classic portal infiltrate consists of a mixed inflammatory infiltrate composed predominantly of mononuclear cells including lymphocytes with many blastic (activated) forms together with eosinophils, macrophages, and lesser numbers of neutrophils. The lymphocytes can be seen surrounding portal vein radicals as well as bile ducts. Portal vein endotheliitis is characterized by lifting up and disruption of the endothelial cells due to lymphocytes marginating and invaginating in between the wall and the endothelium and subsequent infiltration of the entire thickness of the wall of the veins. Bile duct injury is a frequent accompaniment and is characterized by disruption of the normal organized columnar lining cells and replacement by epithelial cells that appear cuboidal with crowding of cells, nuclear overlap, variation in size of nuclei, mitoses, and frequent epithelial cell cytoplasmic vacuolation. Lymphocytes may frequently be seen in between epithelial cells with accompanying apoptosis of both epithelial cells and lymphocytes. These changes can frequently be highlighted using a PAS or trichrome stain, as well as stain for cytokeratins, such as cytokeratin AE1/AE3 or CK7. The inflammation may expand portal areas and in severe cases infiltrate into adjacent lobules with evidence of interface hepatocyte injury. Plasma cells are usually not a feature of ACR but can be seen in some cases of late ACR. Single cell hepatocyte necrosis or confluent periportal hepatocyte necrosis is not a common feature of ACR.
Centrilobular Changes: Involvement of the central veins in the form of extravasation of erythrocytes, lymphocytic infiltration of the wall with lifting up of endothelial cells (endotheliitis), and destruction of endothelium is a common feature of ACR in children. While classically seen accompanying portal inflammation in cases of severe ACR, isolated central perivenulitis is often seen early in pediatric allografts (Abraham et al. 2008). In these instances, there is frequent elevation of AST, ALT, and especially GGTP with persistent elevation in GGTP over a period of time. Most severe pericentral injury leads to single cell hepatocyte dropout and even confluent hepatocyte necrosis. Resolution of central perivenulitis may be manifest as collections of ceroid-laden macrophages as well as pericentral fibrosis in severe cases.
Lobular Changes: The effect of ACR on the lobules is usually a secondary phenomenon related to the degree of portal and centrivenular changes. In some cases hepatocyte dropout may be a prominent feature, and isolated lymphocytes may be seen within sinusoids giving a mild hepatitic pattern to the overall picture. There may be evidence of hepatocyte glycogen accumulation as well as instances with more globular inclusion-like appearance (polyglucosan-like inclusions) of the cytoplasm. Some variation in nuclear size may also indicate an element of regeneration within the lobules.
15.1.4.2.2 Grading of ACR
In order to achieve a uniform assessment of allograft ACR, an international group of liver experts met at the third Banff Conference on allograft pathology and devised a grading system for liver ACR (Demetris et al. 1997a, 2002; Ormonde et al. 1999) (Table 15.2). This classification is now uniformly applied to all allografts, including adult and pediatric cases, although it may not always be applicable in individual situations. It takes into consideration the portal inflammation, including the degree of inflammation, the extent of involvement of the biopsy specimen, and the centrilobular changes. The four main categories included indeterminate, mild, moderate, and severe ACR. While the latter three grades of rejection have well-defined criteria, it is imperative to exclude non-rejection causes in a biopsy before a diagnosis of indeterminate rejection is made. The Banff group also came up with a scoring index called “the rejection activity index” (RAI) (Table 15.3), similar to the scoring used for chronic hepatitis. This takes into consideration the three specific findings of rejection, namely, portal inflammation, bile duct damage, and venular inflammation, giving each a semiquantitative score on a zero to three scale. In many situations, the RAI score appears to be a better indicator of activity of rejection than the grading alone (Horoldt et al. 2006).
Table 15.2
Banff classification for acute cellular rejection
Grade | Features |
|---|---|
Indeterminate | Rare portal areas with infiltrate |
Infiltrate not typical of ACR infiltrate | |
No endotheliitis | |
Mild | Some portal areas involved by classic mixed infiltrate |
Portal venous radicals with endotheliitis | |
No central perivenulitis | |
Moderate | Most portal areas expanded by rejection-type infiltrate |
Severe | Most/all portal areas with extensive infiltrates AND |
Central perivenulitis in few/many lobules | |
Pericentral hepatocytes dropout | |
Portal infiltrates extending into lobules with hepatocytes damage |
Table 15.3
Banff schema for grading liver allograft acute rejection – rejection activity index
Category | Criteria | Score |
|---|---|---|
Portal inflammation | Mostly lymphocytic inflammation involving, both not expanding, a minority of triads | 1 |
Expansion of most or all triads by a mixed infiltrate containing lymphocytes with occasional blasts, neutrophils, and eosinophils | 2 | |
Marked expansion of most triads by a mixed infiltrate containing numerous blasts and eosinophils with inflammatory spillover into the peripheral parenchyma | 3 | |
Bile duct inflammation and damage | A minority of the ducts are cuffed and infiltrated by inflammatory cells and show only mild reactive changes such as increased N to C ratio of epithelial cells | 1 |
Most or all ducts infiltrated by inflammatory cells. More than an occasional duct shows degenerative changes such as nuclear pleomorphisms, disordered polarity, and cytoplasmic vacuolation | 2 | |
As above for 2, with most or all ducts showing degenerative changes or focal luminal disruption | 3 | |
Venous endothelial inflammation | Subendothelial lymphocytic infiltration involving some, but not a majority, of the portal and/or hepatic venules | 1 |
Subendothelial infiltration involving most or all of the portal and/or hepatic venules | 2 | |
As above for 2, with moderate or severe perivenular inflammation that extends into the perivenular parenchyma and is associated with perivenular hepatocyte necrosis | 3 |
In many institutions, protocol biopsies are frequently performed for assessment of liver rejection, but these may not be necessary since LFTs guide the physician’s approach for need of biopsy (Kakizoe et al. 1990a). In several situations, biopsies for altered liver enzymes are performed after empiric treatment of ACR with either steroid bolus or increase in immunosuppressive drugs. In these situations, the histology is altered by the treatment effect and may show much lesser degree of portal cellular infiltrates. Portal endotheliitis and bile ductular damage, however, persist and are clues to the diagnosis of rejection. The centrilobular changes tend to persist for a long time and may be evident in biopsies despite institution of therapy prior to the biopsy.
15.1.4.2.3 Differential Diagnosis
As is evident, ACR can occur at any stage posttransplant and, hence, comes into differential diagnosis for any of the other complications. The characteristic finding of a mixed portal infiltrate of blastic lymphocytes and eosinophils, with few neutrophils together with ductular injury, portal and central vein endotheliitis, and central perivenulitis form the classic pattern of ACR. Reperfusion injury is in the differential diagnosis in the first 2 weeks posttransplant and is characterized more by centrilobular changes of ballooning and cholestasis and lack of inflammatory infiltrate as seen in ACR.
Biliary strictures and obstructive changes need to be differentiated since they can sometimes coexist with ACR (Table 15.4). The classic pattern of biliary obstructive changes is predominantly portal changes with edema, predominance of neutrophils in the infiltrate causing cholangitis and pericholangitis, lack of lymphocytic infiltrate, and cholestasis with progressive peribiliary fibrosis. The above biliary findings in addition to portal and central vein inflammation and mixed portal infiltrate should warrant a combined diagnosis of ACR and biliary obstruction. In this situation, imaging of the biliary tree is required to confirm the diagnosis and treat the biliary issue.
Table 15.4
Differential diagnosis for liver ACR
Histological feature | ACR | Biliary obstructive | EBV hepatitis | Autoimmune hepatitis |
|---|---|---|---|---|
Portal infiltrate | Mixed with lymphocytes, eosinophils, neutrophils, macrophages | Neutrophils predominate | Lymphocytes predominate | Lymphocytes and plasma cells |
Portal edema | ± | +++ | – | – except for PSC |
Bile duct damage | +++ | + | – | + |
Biliary proliferation | ∓ | +++ | – | −/++ in PSC |
Portal venulitis | +++ | – | ∓ | – |
Lobular infiltrates | ± | – | +++ | ++ interface mainly |
Central venulitis | + to +++ | – | + | ++ |
Pericentral dropout | ++ | – | + | + |
EBER | – | – | + | – |
Serologies | – | – | EBV | ++ |
A hepatitic pattern on biopsy should always raise the possibility of an infectious etiology such as EBV hepatitis, which is also characterized by portal infiltrates of lymphocytes, including blastic forms. Spotty necrosis of lobular hepatocytes may be noted as an evidence of central perivenulitis. It is recommended that an EBER-1 probe be performed in these situations to exclude the possibility of EBV hepatitis. The finding of even one or two EBV-positive cells in a given liver biopsy would warrant the diagnosis of EBV hepatitis rather than ACR. Correlation with EBV PCR is required. The lack of classic portal mixed infiltrate should make ACR less likely as a diagnostic possibility in this situation. It is unusual for ACR to coexist in the same biopsy as EBV hepatitis, and the former diagnosis should be made with extreme caution since the treatments are opposite of each other.
A hepatitic pattern which shows a predominance of interface activity with predominance of plasma cells instead of the typical mixed infiltrate should raise the possibility of either recurrent autoimmune hepatitis (AIH), in cases where AIH is the primary disease, or in rare instances de novo AIH in the allograft (Balan et al. 1999; Demetris and Sebagh 2008). Central perivenulitis can be a feature of both (Demetris 2001).
As mentioned before, isolated perivenulitis is most often due to ACR in the pediatric population and can lead to pericentral fibrosis. In such cases, ischemic injury and viral and autoimmune hepatitis can come into the differential diagnosis. Central perivenulitis has been associated with EBV hepatitis as well as cases of autoimmune hepatitis; however, the most likely cause for central perivenulitis remains rejection. It tends to be more resistant to treatment and does increase the risk of progression to chronic rejection (Demetris 2001; Abraham et al. 2008).
Another feature that has been seen in liver transplant patients is the appearance of Lafora body-like cytoplasmic inclusions in hepatocytes. While the differential diagnosis for these ground glass inclusions includes hepatitis B, type IV glycogen storage disease, fibrinogen deficiency, and Lafora body disease, similar changes have been reported following drugs such as cyanamide aversion therapy in adults or even after mycophenolate mofetil in some transplant patients. These are thought to be “polyglucosan” bodies seen in some animal models and are likely the result of altered glycogen metabolism in the liver due to therapeutic agents. We have seen similar changes in patients on FK506 (tacrolimus) therapy alone or with steroids. The inclusions are PAS positive and have a pale eosinophilic to amphophilic appearance as well as an intense eosinophilia in other cases. They tend to persist in multiple sequential biopsies and in some patients are unrelated to modulation of FK506 therapy (Lefkowitch et al. 2006; O’Shea et al. 2007).
15.1.4.3 Late Acute Rejection
The Banff group has documented the occurrence of late acute cellular rejection in long-term survivors with normal or near-normal liver function tests on routine protocol follow-up biopsies (Demetris et al. 2006a). It is usually characterized by the presence of few blastic lymphocytes, more necroinflammatory type interface activity, less venous subendothelial inflammation, and higher incidence of perivenular inflammation with slightly more lobular activity. This gives the biopsy an appearance of acute hepatitis and brings into consideration other causes such as AIH and viral hepatitis. Isolated centrilobular rejection appears to be a frequent mode of manifestation of late ACR. Aggressive therapy is warranted in these cases as persistence of these changes increases the risk of chronic rejection.
The Banff working group has also set criteria for causes of late allograft dysfunction post liver transplant. While some of the changes may appear after the first year, they are usually evident in livers 5 years posttransplant. They document the occurrence of recurrent AIH (30 %), de novo AIH (<5 %), recurrent HBV (100 %), recurrent HCV (100 %), recurrent PSC (20–30 %), acute rejection (<30 %), chronic rejection (3 %), and idiopathic hepatitis (50–60 %) and hence emphasize the importance of close monitoring with either liver enzymes or by annual protocol biopsies where indicated (Demetris et al. 2006a).
15.1.4.4 Assessment of Late Posttransplant Biopsies
Another important finding in children with long-term liver allografts appears to be the presence of fibrosis in both portal and central venular areas. This is especially important in those patients who are being considered for complete withdrawal of immune suppression following a stable clinical course posttransplant (Mazariegos 2011; Sindhi et al. 2002; Mazariegos et al. 1997b; Banff Working Group 2012). Trichrome and cytokeratin stains are important in this situation since the presence of sinusoidal fibrosis may reflect an ongoing subclinical centrilobular rejection, which may need active treatment rather than withdrawal of immune suppression. Portal fibrosis may be a reflection of low grade biliary injury due to rejection or biliary obstruction. Such incidental findings in biopsies are especially apparent in children during the adolescent age group due to the issue of noncompliance with medications. This has become a standard assessment in long-term survivors on sequential surveillance biopsies and helps direct therapy in these children. It has been shown that children in whom immunosuppressive agents are withdrawn lack circulating donor-specific antibodies (Girnita et al. 2010).
15.1.4.5 Chronic Rejection (Fig. 15.7)
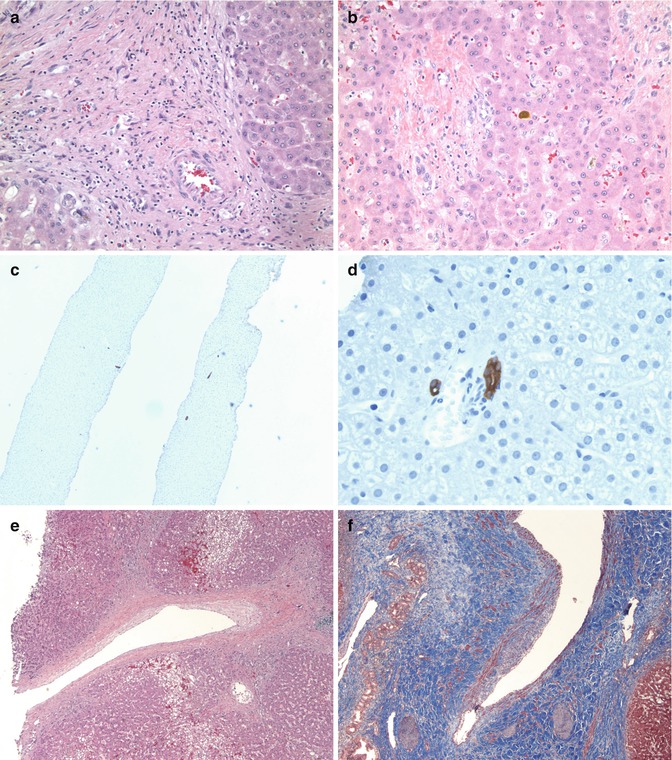
Fig. 15.7
Chronic rejection – liver Tx: (a) Late allograft showing expanded portal area with inflammatory cells, portal vessels with no well-defined interlobular bile duct without any significant ductular reaction (HE ×200). (b) Another portal area showing no expansion but vessels and a damaged bile duct with cuboidal cells and irregular arrangement of lining cells suggestive of ongoing biliary injury and loss. Note the cholestasis (HE ×200). (c) A cytokeratin AE1/AE3 stain highlighting few bile ducts in portal areas without significant ductular proliferation and ductopenic rejection (×40). (d) High magnification highlighting the ductular injury with overlapping of nuclei and crowding of biliary epithelial cells, changes of early ductopenia (AE1/AE3 ×400). (e) Larger hilar vessels showing fibrointimal proliferation, the main feature of graft vasculopathy (HE ×40). (f) A trichrome stain highlights the intimal thickening and luminal narrowing of the artery (Masson trichrome ×100)
Recent advances in operative techniques, imaging, and immunosuppressive treatments have helped prolong graft survival in children and delay the onset of chronic allograft rejection. Chronic rejection has been defined as a largely indolent but progressive form of allograft injury characterized primarily by fibrointimal hyperplasia of arteries or obliterative vasculopathy and loss of small bile ducts (Demetris et al. 1997b; Demetris 1990). The two main forms of chronic rejection in a liver allograft, therefore, is a form of chronic graft vasculopathy and a ductopenic rejection (Blakolmer et al. 2000; Demetris et al. 1987, 1995; Oguma et al. 1989). Although the two components most often occur together, they may be difficult to diagnose on needle biopsies since the biliary changes may be the only findings on a needle core. Clinical and laboratory parameters show a progressive cholestatic pattern of injury with elevations of alkaline phosphatase and GGTP. It is thought that chronic rejection is seen in about 20 % of long-term liver allograft recipients (Blakolmer et al. 2000; Demetris et al. 1998). It appears to be an irreversible end point and is a major cause for re-transplantation (Jain et al. 2000; Quiroga et al. 1991; Marangoni et al. 2008). While typically a disease of long-term liver allografts, we have encountered a rare instance when ductopenia is noted within the first year posttransplant that persists through biopsies and warrants early re-transplantation due to graft failure.
In a study of 166 patients, only four were reported to have histopathology consistent with chronic rejection among the patients on tacrolimus-based immunosuppression. This is considered to be much lower than that seen in the cyclosporine era among pediatric liver allografts (Jain et al. 2003). The most important causes of chronic allograft rejection are unresolved episodes or multiple episodes of acute cellular rejection. Other contributing factors include pre-transplantation circulating donor antibodies, AMR, CMV infection, and ischemic injury.
15.1.4.5.1 Pathology
CR is histopathologically defined by two main features – severe damage and loss of small (less than 60 μm) bile ducts and obliterative arteriopathy. Inflammation is often inconspicuous, and consists mainly of lymphocytes, plasma cells, and macrophages. Intraepithelial lymphocytes may, however, be seen in between degenerating biliary epithelial cells that can show irregular cellular arrangement, eosinophilic transformation of their cytoplasm, and ducts that are only partially lined by epithelial cells. The infiltrate is mainly a T-cell infiltrate with both CD4 and CD8 subsets. As the disease progresses, smaller caliber bile ducts may be missing in several portal areas, and there is associated cholestasis without significant ductular proliferation. The changes may not be conspicuous on light microscopy and may need cytokeratin stains to highlight both the bile duct injury and the loss of bile ducts in individual portal tracts. The cytokeratin stains also highlight the lack of reactive bile ductular proliferation at the interface. The presence of ductular reaction would suggest either an element of recurring or coexistent biliary obstruction. The portal changes may be accompanied by lobular changes such as centrilobular canalicular cholestasis, clusters of intra-sinusoidal foam cells, hepatocyte ballooning, single cell hepatocyte necrosis, and perivenular fibrosis. Active central venulitis is not a feature of chronic rejection but may indicate coexisting ACR.
The second feature of obliterative arteriopathy is typically not recognized on biopsy specimens and is preferentially seen in allograft resections since the vessels involved are usually the large vessels at the hilum. The classic feature is the presence of foam cell arteriopathy with secondary intimal hyperplasia, myxoid change, and luminal narrowing of arteries. Inflammatory infiltrates in the vessel wall, smooth muscle hyperplasia, disruption of the elastic lamina, and adventitial inflammation may also be present. Adjacent large bile ducts at the hilum may show epithelial sloughing, focal necrosis, peribiliary fibrosis, and acute and chronic inflammation.
Chronic rejection has been staged into early and late CR, wherein the early changes usually involve less than 50 % of the portal areas and also show early arterial inflammation without any luminal compromise. Fibrosis around central veins is also minimal. It is thought that early changes tend to persist for a long time in some situations and can be seen to persist over several biopsies. In other situations, the early changes of CR can rapidly progress into changes of late CR. This stage is considered irreversible, and re-transplantation may be the only option for treatment of late chronic rejection.
15.1.4.5.2 Differential Diagnosis of CR
The differential diagnosis for duct injury and ductopenia may be obstructive cholangiopathy, hepatic artery thrombosis or narrowing, drug reactions, and CMV infection. Obstructive cholangiopathy or duct injury usually results in biliary epithelial injury that is associated with ductular reaction, a feature not seen in CR. Hepatic artery thrombosis can cause ischemic injury to the parenchyma as well as lead to development of biliary strictures resulting in a biliary obstructive pattern of injury. CMV infection usually causes lobular hepatitis with identification of viral inclusions but can also result in biliary injury and ductopenia, in which case it is almost impossible to differentiate from CR. Rare instances of recurrent primary sclerosing cholangitis may mimic chronic rejection, but features of cholangitis and pericholangitis with neutrophils and lymphocytes infiltrating into the bile ducts and surrounding them, peribiliary concentric inflammation, and portal edema help differentiate PSC from chronic rejection.
The treatment of CR appears to be similar to that of ACR for it is presumed that ACR must be involved in progression of the disease. Steroid boluses and increased immunosuppressive therapy are needed to try and reverse the injury or at least stabilize it so as to prevent the need for re-transplantation. In those cases where ductopenia affects greater than 50 % of the liver parenchyma, the changes may have reached an irreversible stage.
15.1.5 Biliary Complications
Biliary tract complications such as anastomotic adhesions, bile leakage, ascending cholangitis, biliary strictures, and obstruction can occur following pediatric liver transplant in up to 35 % of cases (Starzl and Demetris 1990a; Demetris et al. 1987). If not detected early, these can lead to biliary cirrhosis and graft loss. Anastomotic strictures appear in the early months following transplantation and are treated by surgical intervention, which decreases the risk of graft loss (Lerut et al. 1988; Anderson et al. 2010). Other biliary strictures could arise as a result of biliary ischemia resulting from any of the above vascular causes or as a result of preformed anti-donor antibodies or reperfusion injury. Living donor grafts, which have become progressively more frequent in children, also have increased biliary complications (Kling et al. 2004). Routine laboratory testing for liver enzymes helps the diagnosis with findings of elevated GGTP and alkaline phosphatase, and subsequently bilirubin. Cholangiography helps confirm the diagnosis in many cases (Meersschaut et al. 2000).
15.1.5.1 Pathology (Fig. 15.8)
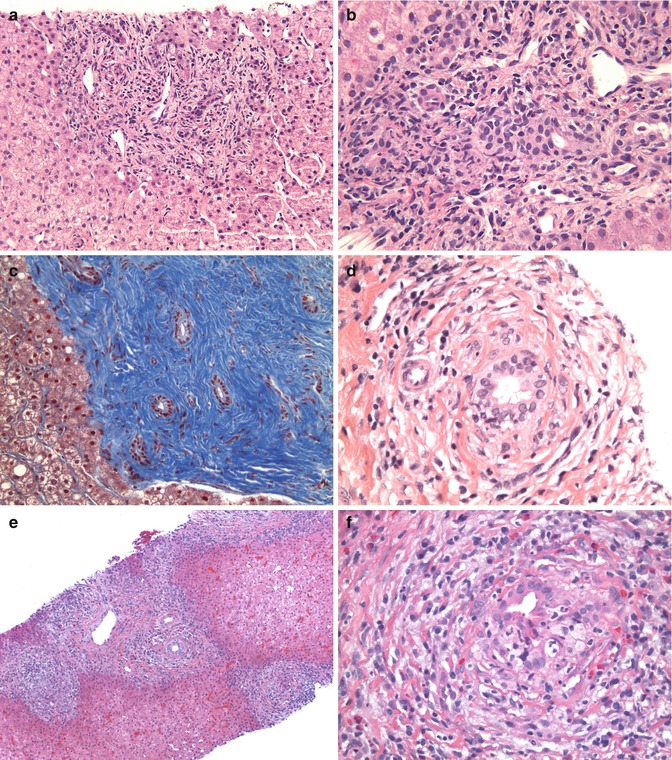
Fig. 15.8
Other complications and changes post liver Tx: (a) Image showing portal expansion with some edema, mixed infiltrates, and mild ductular proliferation (HE ×100). (b) The infiltrate is mixed and includes neutrophils and lymphocytes, with neutrophils infiltrating bile ducts (lower left of image) as well as within the lumen. Note the reactive ductular proliferation (HE ×400). (c) A trichrome stain highlights chronic biliary changes with dense collagen deposition around bile ducts with scant inflammation (Masson trichrome ×100). (d) More typical biliary obstructive feature with peribiliary concentric edema and inflammation with bile duct injury (HE ×400). (e) A case of recurrent primary sclerosing cholangitis in the graft with the ducts showing concentric inflammation and edema around them. Note also the presence of an interface hepatitis (HE ×100). (f) High magnification image of (e) showing the characteristic sclerosing cholangitis changes (HE ×400)
Biliary strictures are manifested in the early phase by portal edema with peribiliary inflammatory cells, including neutrophils and macrophages and evidence of bile duct injury. This injury may be in the form of attenuation of the biliary lining epithelium with overlapping of nuclei, cuboidal shape instead of a more columnar appearance, vacuolation of the cytoplasm, and intraepithelial and luminal neutrophils with or without cholestasis. Ductular proliferation is seen as the disease progresses, and portal fibrosis may be an end result. In late cases, concentric peribiliary fibrosis may be seen and mimic the changes of sclerosing cholangitis.
15.1.5.2 Differential Diagnosis
Some of these histological changes overlap with those seen in ischemia-reperfusion injury and also need to be differentiated from acute cellular rejection (Table 15.4). Other differential diagnoses that may arise include recurrence of primary disease such as primary sclerosing cholangitis, which may need serological investigation to confirm the diagnosis (Nakamura et al. 2005; Lerut et al. 1988).
15.1.6 Infections
The next major group of posttransplant complications is infections. They may be due to any organism and can occur at any time in the postoperative period. While bacterial infections and sepsis are seen early, fungal infections and viral complications usually occur in the first few weeks or beyond. It is always useful to consider infections as a possibility while reviewing any allograft biopsy (Table 15.5).
Table 15.5
Salient features of post-liver-transplant infections
Adenoviral hepatitis: Punched-out necrotic foci |
Smudged nuclei of hepatocytes with nuclear inclusions |
Adenovirus immunostain positive |
Infiltrates of neutrophils, macrophages, lymphocytes |
Occasionally ascending biliary infection with bile duct involvement by virus |
Herpes hepatitis: Punched-out hepatic necrotic foci in parenchyma |
Rare cases of diffuse massive necrosis |
Herpes inclusions with multinucleation seen |
Immunostain positive for herpes inclusion and antigen in necrotic foci |
Neutrophilic, macrophage, lymphocytic infiltrate |
CMV hepatitis: Small pockets of parenchymal neutrophilic infiltrates |
Associated single cell hepatocytes necrosis |
Infiltrates in portal areas, rarely extensive |
CMV nuclear and cytoplasmic inclusion occasional or prominent |
Immunostain may show more positive nuclei including non-transformed cells |
Bacterial and fungal hepatitis: |
Abscess formation or diffuse lobular neutrophilic infiltrate |
Fungal organisms may be identified by Grocott stains |
Blood cultures positive for bacterial sepsis |
Intracellular and canalicular cholestasis with portal infiltrates |
15.1.6.1 Bacterial and Fungal Infections
One of the complications of prolonged immunosuppression in transplant patients is the occurrence of bacterial infections. In the early posttransplant period, wound infections are common and can lead to dehiscence of the wound or exudation from the site of incision. Sepsis due to any bacterial infection can be caused by intravenous line infections or wound infections and lead to inflammation within the liver parenchyma that may be composed of both acute and chronic inflammatory cells as well as granulomas. They can occur at any time posttransplant and manifest as fevers, abdominal pain, and alterations of liver functions (Demetris et al. 1987; Jaffe and Yunis 1989; Hollenbeak et al. 2003; Nafady-Hego et al. 2011). Canalicular cholestasis and ballooning of hepatocytes along with a lobular hepatitic pattern with neutrophils in sinusoids and single cell hepatocyte necrosis may be features of bacterial sepsis. In rare instances, parenchymal abscesses may be seen either due to bacterial or fungal infections. It is always helpful to do special stains for either bacteria (Gram’s) or fungal organisms (Grocott stain) to exclude causative organisms. Rare instances of mycobacterial infections have also been reported (McDiarmid et al. 1995). Microbiological studies also need to be done on these biopsies, and it is preferable to obtain the specimens fresh so that cultures can be performed.
15.1.6.2 Viral Infections (Fig. 15.9)
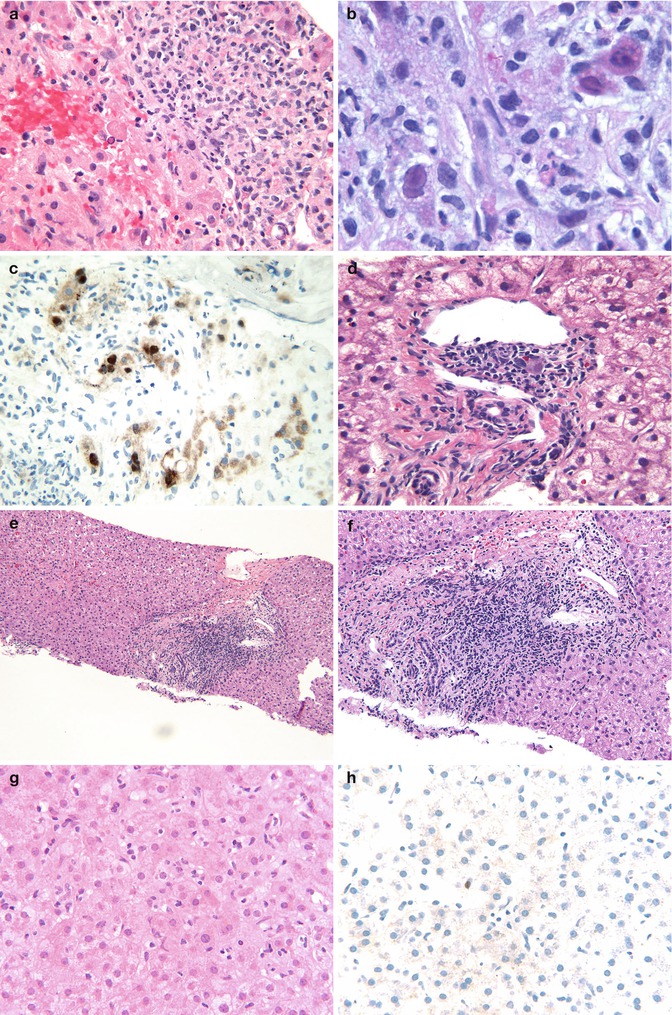
Fig. 15.9
Viral infections post liver Tx: (a) Liver biopsy showing areas of coagulative necrosis with a mixed portal infiltrate. Note scattered cells with a smudged nucleus suggestive of viral inclusions (HE ×400). (b) Oil immersion view highlighting the viral inclusions of adenovirus (HE ×1,000). (c) An immunostain for adenovirus highlights the many inclusions in the portal area and lobules (IHC-adenovirus ×400). (d) A portal area showing scattered inflammatory cells and an inclusion of CMV (HE ×400). (e) Liver biopsy showing portal expansion by a lymphoid infiltrate in a patient with hepatitis C infection (HE ×200). (f) Higher magnification of same showing the predominantly lymphocytic infiltrate with some interface activity (HE ×200). (g) Liver biopsy showing a hepatitic pattern with sinusoidal lymphocytic infiltrate (HE ×400). (h) An EBER-1 probe showing a single positive cell in the lobule suggestive of EBV infection (ISH-EBER-1 ×400)
The most common viral infections in a posttransplant setting in children include cytomegalovirus (CMV), adenovirus, herpes simplex virus (HSV), or varicella zoster virus (VZV) and Epstein-Barr virus (EBV). These infections are much more common in pediatric patients especially since children may not have been exposed to these viruses and, hence, are susceptible to primary infection, which results in a more severe form of disease. In some situations following infections, prolonged shedding of the viral particles may be seen as a manifestation of latency or chronic carrier status. Monitoring of viral PCRs is extremely useful to confirm the diagnosis and is now a readily available tool in most transplant centers.
15.1.6.2.1 Cytomegalovirus
CMV infections develop between 3 and 8 weeks posttransplant, especially when the immunosuppressive drug dosage has been increased to treat ACR (King et al. 1990; Green 2002). CMV infections are a risk factor for hepatic artery thrombosis in children and can also lead to biliary strictures. Any organ can be infected depending on the extent of viral dissemination, but the liver and gastrointestinal tract are commonly involved. CMV hepatitis is characterized by spotty lobular hepatocyte necrosis with Kupffer cell activation and patchy lobular inflammation characterized by small aggregates of neutrophils and macrophages forming microgranulomas. Infected hepatocytes as well as endothelial cells and bile ducts show the characteristic large eosinophilic intranuclear inclusions accompanied by small amphophilic to basophilic cytoplasmic inclusions. This is usually associated with elevated CMV viral load that can be monitored by PCR. Immunohistochemical staining with antibody to CMV early antigen highlights the many transformed cells as well as hepatocyte nuclei that contain viral antigen and are still not transformed. An antibody to the late antigen is also available and typically stains the viral inclusions seen within large transformed cells. Newer antibodies that detect both components are now available and a useful adjunct tool in suspected cases. The treatment for CMV infection usually involves a decrease in immune suppression as well as therapy with ganciclovir or other antivirals. In most instances, CMV infections can be effectively treated without any effect on graft function. Uncontrolled CMV infection results in progressive ductular injury and can result in ductopenia and mimic or hasten CR.
15.1.6.2.2 Adenoviral Infection
Michaels et al. have reported an incidence of adenoviral infection after pediatric liver transplantation to be approximately 10.1 % in their series (Michaels et al. 1992). The median time for transplant adenoviral infections is approximately 25 days, and almost all infections are seen within the first 3 months. The most common adenoviral serotypes are 1, 2, and 5, of which serotype 5 causes adenoviral hepatitis (Jaffe and Yunis 1989; Demetris et al. 1987; Brundler et al. 2003). Adenoviral latency is also possible and can lead to reactivation of infection in later stages posttransplant. Histologically, they give rise to pockets of necrosis surrounded by macrophages and neutrophils causing punched-out lesions (pox-like) within the parenchyma. These necrotic zones are frequently seen in the periportal areas. Viral inclusions are usually seen within hepatocytes as intranuclear smudged inclusions surrounding the necrotic zones. This can be demonstrated with an immunohistochemical stain for adenovirus. In rare instances, adenoviral hepatitis can manifest as fulminant liver failure posttransplant. Another form of adenoviral infection frequently seen in pediatric allografts is a form of ascending cholangiohepatitis (Brundler et al. 2003). In this situation there is usually a prominent cholangitis with adenoviral inclusions seen within biliary epithelial cells. There is also an associated necrotizing hepatitis. There is usually concomitant adenoviral infection of the gastrointestinal tract in these patients. Treatment for adenoviral infections of the liver would include antiviral therapy with decrease in immunosuppression. Severe cases causing fulminant hepatic failure would warrant re-transplantation. Adenoviral infections have not been reported to date in re-transplants, and, hence, is not a contraindication for re-transplantation (Green 2002).
15.1.6.2.3 HSV and VZV Infection
Herpetic infections of liver are less common but can be seen posttransplant (Green 2002; Jaffe and Yunis 1989). It leads to submassive or massive hepatic necrosis similar to that seen in neonatal herpes or in non-immunosuppressed patients. Histologically, it is characterized by confluent areas of necrosis that may be surrounded by rare hepatocytes showing nuclear clearing or multinucleation characterized by viral cytopathic change. An immunohistochemical stain for HSV can detect viral inclusions and frequently shows antigen localized within the necrotic areas. Treatment is by complete withdrawal of immunosuppression and treatment with antivirals.
15.1.6.2.4 Epstein-Barr Virus (EBV) Infection
EBV infection is common in children and is usually due to seroconversion after transplant in previously unexposed recipients (primary infection) or in some instances may be related to reactivation of the virus following immunosuppression (Jaffe and Yunis 1989; Green 2002). The frequency of EBV infection in 92 pediatric liver recipients was reported to be 63 % by Ho et al. (1988). Of these, 49 % were seronegative and 75 % of these acquired primary infection. The diagnosis of primary EBV infection is made on the basis of development of IgG antibodies against viral capsid antigen (VCA). The presence of IgM anti-VCA titer, the absence of anti-Epstein-Barr nuclear antigen (EBNA), and the presence of a heteroagglutination titer are diagnostic of primary infection. More recently EBV PCR is routinely monitored in the blood, and a rising titer is indicative of an infection, especially if the viral PCR is in the millions (Lee et al. 2005; Spada et al. 2001). The significance of detecting EBV infection is to prevent the development of EBV-related malignancies in the posttransplant setting. This requires prompt reduction of immune suppression and use of antiviral drugs (Lee et al. 2005; McKnight et al. 1994; Renard et al. 1991). The most common clinical presentation is with fevers, sore throat, jaundice, and lymphadenopathy constituting the classic infectious mononucleosis-like picture.
Pathology
EBV hepatitis is characterized by a diffuse lobular infiltrate that is associated with central perivenulitis as well as portal infiltrates composed of plasma cells, lymphocytes, including blastic lymphocytes, and scattered macrophages. Eosinophils and neutrophils are not a feature of the infiltrate. A PAS stain highlights the lobular activity with Kupffer cell activation and may rarely show single cell hepatocyte necrosis. An EBER-1 probe is usually helpful in differentiating the hepatitic infiltrate of EBV infection from other causes (Randhawa et al. 1992). The presence of even a single positive-stained lymphocyte within the lobule in a liver biopsy would favor an EBV infection in the presence of an elevated EBV PCR in the blood. In portal areas, EBV-positive lymphocytes are also indicative of infection unless present within a lymphoid follicle, where a possibility of latent infection may arise.
In those situations where EBV infection has progressed to lymphoproliferative disorders (Fig. 15.10), large confluent aggregates of polymorphic or even monomorphous lymphoid cells may be seen expanding portal areas and, in many instances, replacing adjacent lobules. It is important to note that both EBV infection and PTLD may be a systemic disease and involve several other organs besides the allograft liver (Ho et al. 1988; Nalesnik et al. 1988). Allograft involvement is less common and when present can give rise to mass lesions that are diagnosed radiologically. An ultrasound- or CT-guided biopsy is attempted, and the histology may show replacement of parenchyma by the mass composed of either a heterogeneous population of cells that include varying sized lymphocytes and plasma cells constituting polymorphous PTLD (pPTLD). Alternatively, the lesion may be composed of a uniform population of large transformed, immunoblastic lymphoid cells with prominent mitoses and high proliferation index, features of a monomorphous PTLD (mPTLD). This PTLD usually has a B-cell phenotype and is easily recognized due to the presence of numerous cells staining positive with an EBER-1 probe which highlights the clonal proliferation. Staining with EBER-1 probe has been used to try and differentiate polymorphous from monomorphous PTLD by the staining of variable types of cells in a pPTLD versus the monotonous stained population in mPTLD. Rare instances of PTLD having a morphology resembling Hodgkin lymphoma have also been reported (Nalesnik et al. 1993). The treatment of PTLD is usually complete withdrawal of immune suppression together with monoclonal antibody therapy and chemotherapy in cases of monomorphous PTLD or lymphoma (see later section on PTLD).
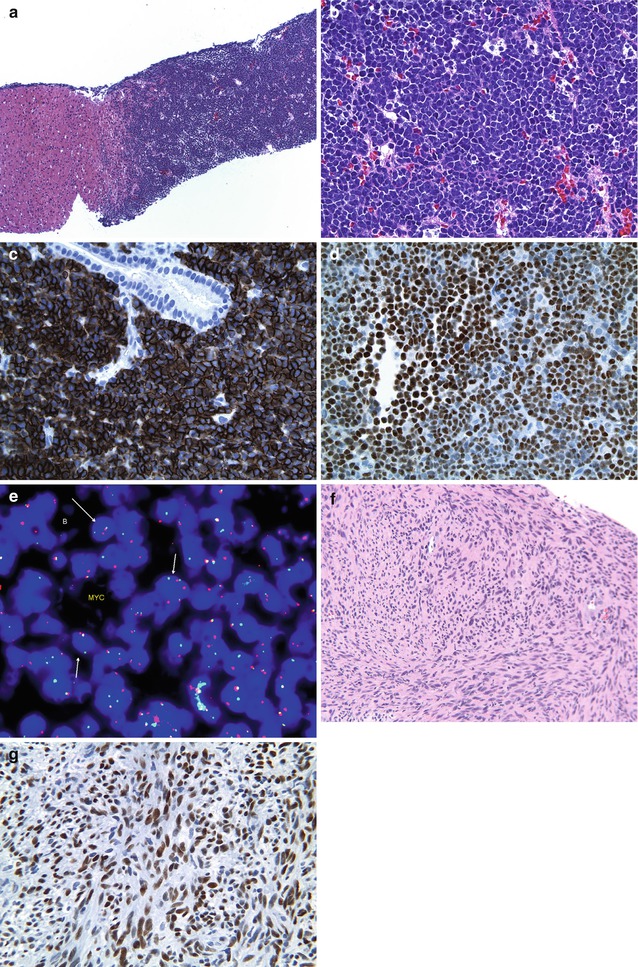

Fig. 15.10
Post-liver-Tx tumors: (a) A liver biopsy showing replacement of the parenchyma by a mass lesion composed of sheets of blue cells (HE ×40). (b) Higher magnification of same case showing uniform population of small to intermediate non-cleaved lymphoid cells suggestive of Burkitt lymphoma (HE ×400). (c) A CD20 stain highlights the clonal B-cell nature of the atypical infiltrate (CD20 ×200). (d) An EBER-1 probe shows diffuse strong staining of the monomorphous population indicating clonality (ISH-EBER-1 ×200). (e) A FISH probe for t(8;14) translocation is positive in this case confirming Burkitt lymphoma. (f) Another example of a post-liver-transplant biopsy showing a mass composed of relatively bland spindle cell proliferation with abundant eosinophilic cytoplasms and oval to spindled nuclei with blunt edges – features of a posttransplant spindle cell tumor (HE ×100). (g) An EBER-1 probe highlighting diffuse strong staining of all the spindle cells (EBER-1 ×200)
15.1.7 Recurrent Disease Posttransplant
The most common diseases that recur posttransplant include viral hepatitis B and C and autoimmune hepatitis. Since the indication for pediatric liver transplant is only occasionally hepatitis B or hepatitis C infection, or related complications, the few instances where hep B or hep C is found posttransplant may represent either primary disease or a recurrence. The clinical and histological features are similar to that seen in viral hepatitis, and serological and PCR viral loads help in confirming the diagnosis.
Autoimmune hepatitis presents a difficult challenge in that it has a high incidence of recurrence posttransplant and can recur at any time during the posttransplant course (Balan et al. 1999; Demetris and Sebagh 2008; Detre et al. 1989; Wright et al. 1992). Serological confirmation may be difficult in these cases due to persistence of positive pre-transplantation serological tests. The histological features are also difficult to recognize due to the overlap with acute cellular rejection and viral infections. A plasma cell-rich infiltrate with interface activity, biliary injury with peribiliary edema and fibrosis, central perivenulitis with single cell hepatocyte necrosis, and absence of evidence of EBV or other viral infection, together would raise this possibility. Recurrent AIH invariably results in rapid graft failure and is one of the indications for re-transplantation. AIH also has the potential to recur in re-transplanted livers.
Primary sclerosing cholangitis also recurs to a similar extent as autoimmune hepatitis and can be difficult to differentiate from biliary obstructive causes such as postoperative ischemic biliary strictures or strictures related to vascular thrombosis (Abu-Elmagd et al. 1993a; Balan et al. 1999; Dvorchik et al. 2002; Lerut et al. 1988). The histology is similar to that of the primary disease (Fig. 15.7g, h). There have been rare instances of recurrent giant cell hepatitis or a recurrent familial intrahepatic cholestasis-BSEP disease, and tumors have recurred in the allograft liver, especially in cases with extrahepatic extension or extensive vascular invasion (Pappo et al. 1994; Siebold et al. 2010). We have also encountered recurrence of Budd-Chiari syndrome in allografts.
15.1.8 Outcome of Liver Transplantation
While there has been a steady increase in the expectancy in survival of liver allografts in the tacrolimus era as opposed to the cyclosporine A era, due to better control of episodes of rejection, there appears to have been an increased incidence of EBV-related PTLD in these patients (Demetris et al. 1992a; Fung et al. 1996; Jain et al. 2000). In order to decrease complications of prolonged immune suppression, attempts at withdrawal of immune suppression for sustained periods of time have been initiated, especially after pediatric liver transplant (Ramos et al. 1995; Mazariegos 2011; Mazariegos et al. 1997a, 2007). Recent advances in identification of specific gene transcripts after adult liver transplant that predict the ability to achieve tolerance raises the possibility of identification of similar mechanisms in children. It has been shown that tolerant patients exhibit a higher incidence of circulating plasmacytoid dendritic cells relative to conventional myeloid dendritic cells (Thomson et al. 2001; Jain et al. 2011). The 20-year actuarial patient and graft survival is 35.8 and 32.6 %, respectively, in a large adult and pediatric cohort of liver transplant recipients under tacrolimus immunosuppression. In that series and others, a significantly better survival was seen among children (Jain et al. 2000, 2011). The 10-year graft survival for children with liver transplants is about 62 % according to the Organ Procurement and Transplantation Network (OPTN) data (OPTN data as of 12/30/2011). The causes of death after pediatric liver transplantation are usually related to liver failure caused either by thrombosis of hepatic artery or primary nonfunctional graft or severe rejection (Jain et al. 2000, 2011). Sepsis and opportunistic infections are other major reasons for posttransplant death (Kashyap et al. 2001; Kahn et al. 1988; Yandza et al. 1992; Soltys et al. 2007). Rare instances of posttransplant neoplasia have also resulted in graft failure and death.
15.2 Pediatric Small Bowel/Multivisceral Transplant Pathology
Small intestinal transplant was first attempted in the 1960s with limited success even in the cyclosporine era. It was not until the 1980s with the advent of tacrolimus that it gained momentum, and the introduction of induction protocols with antilymphocyte antibodies appears to have improved the outcome of these grafts (Starzl 2000, 2001). Small bowel transplants are now being done with increasing frequency in children suffering from intestinal failure for a variety of diseases (Kocoshis 1994). The biggest factor in determining the need for intestinal transplantation appears to be the degree of dependency on total parenteral nutrition (TPN), which has its associated complications including infections and liver failure. In most situations, the transplantation is usually for a short gut syndrome as a result of prior surgeries for various congenital defects and conditions. The complications following small bowel transplant (SBT) are to some extent similar to those of liver transplant but have unique features without much of the surgical difficulties associated with a liver transplant. The survival of patients with SBT has also improved in the tacrolimus era, as compared to the cyclosporin era. Two types of intestinal transplant procedures are performed: one consisting of isolated small bowel and the second combining small bowel and liver transplantation. A third type of surgery is one in which the small bowel is a component of a multivisceral transplant, consisting of a combination of small bowel, large bowel, portions of duodenum, and stomach, including pancreas and liver (Jaffe et al. 1989). While overall improvement in postoperative care and pretransplant preparation have contributed to increased survival of grafts, the type of surgical procedure plays a role in that there appears to be a consistently better outcome for liver/SBT patients compared to SBT alone. This is thought to be related to relatively easier vascular anastomoses and the availability of collaterals in the combined versus the isolated SBT patients. Overall, rejection seems to be the main postoperative complication following SBT. Close monitoring in the immediate postoperative period along with regular endoscopic examination and protocol biopsies has been recommended to improve the overall outcome. Several recent reviews and chapters highlight the pathology of SBT in adults and children (Husain et al 2010; Ruiz 2009; White and Ranganathan 2011).
15.2.1 Indications for Pediatric Small Bowel/Multivisceral Transplant (Table 15.6)
Table 15.6
Indications for pediatric small bowel transplant at CHP
Short gut syndrome | |
Gastroschisis | 22.1 % |
Intestinal atresia | 6.0 % |
Intestinal volvulus | 17.3 % |
Necrotizing enterocolitis | 10.8 % |
Resection-tumor | 0.8 % |
Resection-arterial thrombosis | 0.4 % |
Others | 5.6 % |
Hirschsprung disease | 6.4 % |
Intestinal pseudo-obstruction | 7.6 % |
Microvillus inclusion disease | 5.2 % |
Tufting enteropathy | 6.0 % |
Graft failure/re-transplantation | 6.0 % |
Others | 5.8 % |
As mentioned above, the main indication for SBT appears to be sequelae of short gut syndrome with associated TPN-related complications, where the timing of transplant is determined by both duration of TPN and associated liver complications (Kaufman et al. 2001). Repeated central vein infections with resultant sepsis may also hasten the need for SBT. The causes of short gut syndrome in the pediatric age group, for which SBT has been performed, include anatomical defects such as gastroschisis and intestinal atresias, mechanical defects such as volvulus, ischemic injury such as necrotizing enterocolitis, and motility defects such as long-segment Hirschsprung disease and intestinal pseudo-obstruction (Ghobrial et al. 2000; Mazariegos et al. 2009; Sigurdsson et al. 1999; Reyes 2001; Iyer et al. 2001). In rare instances, intestinal resections for familial polyposis may also result in short gut syndrome (Reyes et al. 1998). The other indication for intestinal transplant is a group of congenital disorders that cause intractable diarrheas in infancy (Ferretti et al. 1996). These include microvillous inclusion disease, where there is a defect in the MYO5B gene that results in defective migration of the microvillous proteins to the surface resulting in absence of the intestinal brush border. This leads to a protein-losing enteropathy due to defective absorptive abilities. The second major cause of intractable diarrhea is tufting enteropathy, which is caused by a defect in the EPCAM gene that results in epithelial discohesiveness and formation of surface epithelial “tufts,” both in the small intestine and large intestine (Lacaille et al. 1998; Paramesh et al. 2003; Ranganathan et al. 2014). Autoimmune enteropathy, which is the third major cause of intractable diarrheas, has also resulted, in rare instances, in SBT. Transplantation for inflammatory bowel disease is still not a major indication in the pediatric age group, but may be done in adults who have had IBD since childhood. Other unusual causes for small bowel transplant include tumors of the mesentery such as a desmoid fibromatosis (Morris et al. 1995).
Contraindications to SBT are few and usually include systemic diseases in which small intestinal disease is only a part of the manifestation such as immunodeficiency syndromes or systemic autoimmune disorders. Similarly patients with neurologic complications or non-resectable malignancies should not be considered for transplant.
15.2.2 Role of the Pathologist
The role of the pathologist in the operating room is limited and is concerned more with workup of the primary disease rather than evaluation of a donor segment. The most likely situation in which a pathologist may be needed in the OR at the time of transplant procedure is in cases of long-segment Hirschsprung disease, where the surgeon needs to know the level at which ganglion cells are present in the recipient bowel to ensure transplantation to a normally ganglionated segment. Rare instances of long-segment Hirschsprung disease that extend into the duodenum and stomach may be an indication for frozen section evaluation of margins. Donor organ evaluation is usually not required in cases of SBT; however, rare instances of incidental primary disease may be found by the pathologist at the time of evaluation of an extra donor pre- or postperfusion loop of bowel sent along with the explanted native specimen. A rare instance of CMV infection in the donor bowel has been encountered by us. It has been the practice in our institution to evaluate postperfusion bowel specimens for evidence of immediate injury to the bowel.
Usually, complications of small bowel transplant are diagnosed on endoscopic allograft ileal biopsies. While most of the biopsies are done in the initial period through the stoma site, in rare situations biopsies from both the lower part of the allograft through the stoma and upper allograft through an upper endoscopy may be performed. The biopsies are usually received in formalin and include two to three fragments of ileal mucosa to provide an adequate sample of crypts for evaluation of rejection. Routine H&E staining suffices in most instances, and immunohistochemical stains may only be necessary where viral infections or abnormal lymphoid proliferations are seen within the biopsy. A trichrome stain for evaluation of vessels may be performed only if a deep biopsy is available in the later stages of the graft evaluation. While the usual practice is to biopsy the SB in isolated SBT, biopsies of allograft colon or stomach may sometimes be required in assessment of multivisceral transplants. A close interaction with the transplant surgeons/treating physicians, which can be daily for difficult cases, is necessary to interpret the histological findings in the appropriate clinical context.
15.2.3 Complications of Small Bowel Transplant
15.2.3.1 Preservation Reperfusion Injury
The process of transplantation involves retrieval of the organ from a cadaveric donor and transport to a remote site in a preservation solution to the institute where the organ is to be transplanted into a recipient. The usual procedure is to do a back table dissection and then anastomose the organ to the recipient’s bowel and unclamp the vascular supply following anastomosis of the vessels. The relative response of different donor organs is different and determined by the time taken to transport and the state of the bowel in the donor. Injury is usually a result of prolonged preservation of the bowel in transport solutions followed by reperfusion in the recipient posttransplant. This results in varying degrees of mucosal injury with bacterial translocation and release of cytokines causing allograft damage (Cicalese et al. 2001; Schweizer et al. 1992; Thaler et al. 1993; Tesi et al. 1996). This is usually a consequence of impairment of vascular flow to the bowel and is usually noted as soon as the donor bowel segment is perfused by blood (Balaz et al. 2007; Nakamura et al. 1994). In most instances, the changes are mild and are only recognized because of routine submission of excess reperfused bowel segments at the time of surgery. Severe injury may be grossly evident as extreme congestion of the bowel segments, necessitating in some instances removal of the graft or a segment of the graft because of irreversible ischemic damage.
15.2.3.1.1 Pathology (Fig. 15.11a, b)
Fig. 15.11
SBT – immediate complications: (a) A day 0 immediate post-Tx donor bowel biopsy showing mucosal injury with regenerative changes, stromal prominence, and congestion, all due to reperfusion injury (HE ×40). (b) Higher magnification of a highlighting the crypt injury with occasional apoptosis and regenerative changes which extend to the surface epithelium. Note mucosal congestion and hemorrhages (HE ×100). (c) A 10-day posttransplant biopsy showing epithelial denudation with prominent capillaries with plump endothelial cells and rare neutrophils in the lumen – clues to suggest AMR (HE ×200). (d) Higher magnification of (c) (HE ×400). (e) A C4d stain showing diffuse strong C4d staining within endothelium of capillaries in the lamina propria (DAB ×200). (f) Higher magnification of (e) showing the endothelial C4d staining (DAB ×400)
Histology shows changes ranging from congestion of capillaries in the lamina propria associated with occasional crypt apoptosis to some regenerative changes within crypts. Inflammatory infiltrates are not a feature in the immediate day 0 (zero) specimens, but may be seen in biopsies from the first week. The infiltrate usually consists of neutrophils and macrophages, some of which may contain hemosiderin pigment. More severe cases are associated with surface epithelial denudation with marked crypt injury and multiple crypt apoptotic activity associated with neutrophilic infiltrates and mucosal hemorrhages (Nakamura et al. 1994; Shah et al. 1997). The changes can be recognized in biopsies usually within the first week posttransplant with regenerative changes persisting for up to 15 days posttransplant in protocol biopsies. It has been suggested that more severe reperfusion injury increases the risk for acute cellular rejection and subsequent graft dysfunction in SBT patients. This is possibly related to the presence of activated macrophages (CD14 +) with subsequent release of cytokines that promote mucosal injury and rejection episodes by cytokine-mediated lymphocyte targeted injury (Ashokkumar et al. 2011).
15.2.3.1.2 Differential Diagnosis
The major differential diagnosis for severe reperfusion injury would be arterial thrombosis or antibody-mediated rejection. Ischemia related to arterial thrombosis usually results in severe graft dysfunction and may warrant removal of the graft. Reperfusion injury rarely results in such severe dysfunction or graft loss. Antibody-mediated rejection (AMR) usually shows dilated capillaries with prominent endothelial cells with neutrophil margination and fibrin platelet thrombi and can usually be diagnosed with the help of a C4d stain. Acute cellular rejection (ACR) is usually readily distinguished from reperfusion injury, but ACR is frequently seen in conjunction with reperfusion injury. Viral infections are usually not seen at this time, but acute bacterial enteritis and sepsis may sometimes occur as evidenced by abundant neutrophilic infiltrates and systemic symptoms.
15.2.3.2 Acute Rejection
Acute rejection is the main complication posttransplant and can occur at any time starting from a few days to months and even years following transplantation. Normally posttransplantation, the recipient’s lymphocytes slowly infiltrate into the donor allograft and progressively colonize the graft. This is a continuous process and can be manifested, in protocol biopsies, as progressive loss of lymphoid follicles in the graft, as well as burnt-out germinal centers. If chimerism studies are performed on the allograft bowel, a mixed population of both donor- and recipient-specific lymphocytes are found (a mixed lymphocyte culture) (Abu-Elmagd et al. 1994b; Tryphonopoulos et al. 2004; Teitelbaum et al. 1994; Li et al. 1993). This is a normal phenomenon and ideally prevents either rejection of the bowel or graft-versus-host disease (GVHD). Any imbalance in this repopulation results in cellular rejection of the bowel. Rejection can be of two types, namely, antibody-mediated rejection (AMR) and acute cellular rejection (ACR). It is thought that the incidence of graft rejection in intestinal transplants is very high, in some series reportedly up to 100 %, due to the presence of native intestinal lymphoid tissue. We have encountered rejection in almost 60–70 % of intestinal transplant patients in children (Nayyar et al. 2010; Mazariegos et al. 2009).
15.2.3.2.1 Antibody-Mediated Rejection (Acute Humoral Rejection)
AMR is usually caused by preformed circulating antibodies to donor antigens and usually results in immediate graft dysfunction. The preformed circulating antibodies are usually the result of prior blood transfusions and may also be encountered in patients undergoing re-transplantation for failed allografts (Fujisaki et al. 1994, 1996). It usually occurs earlier post transplant and can be seen within hours to days posttransplant and rarely may take up to a few weeks to develop. It may occur independently or may coexist with acute cellular rejection (Murase et al. 1994; Abu-Elmagd et al. 1993b). Postperfusion, the allograft usually becomes cyanotic and shows petechial hemorrhages which may resolve in a short time. However, presensitized children may develop a full-blown picture in a few days with mucosal ulceration and congestion visualized on endoscopy.
Pathology (Fig. 15.11c–f)
Mucosal biopsy shows severe congestion with neutrophilic margination within the capillaries that are dilated and have plump endothelial cells, as well as fibrin platelet thrombi in the superficial vessels. Deeper submucosal vessels are rarely represented in the specimen and, if present, may show evidence of some vasculitis or arteritis. The lamina propria appears more edematous and shows hemorrhages. Lymphocytic infiltration is not a feature and, if present, should raise the possibility of a concomitant ACR. Similarly, crypt apoptosis is also uncommon and would also raise the possibility of coexistent ACR. Severe cases may show mucosal ulceration. The diagnosis can usually be confirmed with C4d stain, which shows diffuse capillary endothelial deposition of C4d in most of the superficial capillaries. It is important to recognize that occasional capillary staining for C4d may sometimes be noted in other inflammatory conditions and by itself does not warrant a diagnosis of AMR. While C4d staining as a marker for AMR has been well established in other organ transplants, their significance in intestinal AMR is still debated (de Serre et al. 2008; Troxell et al. 2006; Fujisaki et al. 1996; Ruiz et al. 2010). Correlation with titers of circulating donor-specific antibodies is necessary to confidently diagnose AMR.
Fujisaki et al. have described severe forms of AMR, in which vasculitis of large and small arterial branches were noted (Fujisaki et al. 1994, 1996). It is possible that this vasculitis may not be detected in biopsy specimens, but would be better appreciated on graft enterectomies, where mesenteric vessels may best show these changes. A grading system for vasculitic lesions in AMR has been suggested by Ruiz et al. depending on the degree of inflammatory changes within the vessel wall (Ruiz et al. 2006). There is a lot of overlap of AMR with hyperacute or accelerated acute rejection. The latter is typically seen at the time of transplant and, in some ways, is related to the presence of preformed antibodies and hence overlaps with AMR. Treatment with antilymphocyte antibodies such as OKT3 may be needed to treat AMR. The treatment is usually continued over a 7-day period with serial monitoring of the donor-specific antibodies as well as endoscopic and pathologic evaluation.
15.2.3.2.2 Acute Cellular Rejection (ACR)
ACR is the most common complication and is encountered routinely in patients with intestinal transplantation. It usually begins early posttransplant but in rare cases can be a later presentation. A clinical diagnosis of ACR is difficult since there are no specific laboratory values to determine or follow, and the clinical presentation of increased stomal output, abdominal pain, or fevers are nonspecific and do not differentiate the various etiologies of graft dysfunction. A mucosal biopsy of the allograft appears to be the only way to diagnose ACR. In patients in whom the stoma has been closed, diarrhea and abdominal cramps may be the symptoms of ACR. Endoscopy may show a normal mucosa even with moderate grades of rejection in a biopsy specimen. Severe rejection is seen endoscopically as ulcers with the caveat that the changes may be focal and sometimes missed. Sufficient endoscopic expertise is required to distinguish the allograft from the anastomotic segment, blind loop, or even native intestinal segment due to the complex nature of the surgery.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








