Gastroesophageal reflux
Allergy
Infections
Ingestion of corrosive agents
Prolonged retention of medication pills
Trauma
Repaired esophageal atresia
Crohn’s disease
Radiation
Motility disorder
2.1.2 Histology
As in other segments of the gastrointestinal tract, the histologic diagnosis of inflammation can be quite subjective because of the incomplete knowledge of the normal variation of mucosal histology. While most authors agree on the characteristics of some features of the normal esophageal mucosa (absence of neutrophils, thickness of the basal cell layer, and length of papillae), the criteria for variables such as the number of intraepithelial inflammatory cells have not been uniformly defined. The normal squamous epithelium includes a basal cell layer that does not exceed 15 % of the entire epithelial layer; the portion of the lamina propria termed papillae may extend into the squamous layer up to 50 % of the epithelial thickness (Dahms 1997; Fenoglio-Preiser 2008). These features, however, can be difficult to assess when the orientation of the biopsy is not optimal. This difficulty is particularly problematic among grasp biopsies, the most common technique used, because those biopsies do not usually include the lamina propria and the epithelium tends to be twisted along the length of the biopsy.
The upper normal values of other components of the esophageal mucosa, such as intraepithelial lymphocytes and eosinophils, are less well defined. Lymphocytes in the normal squamous mucosa can adopt two different morphologic appearances: typical cells with round nuclei or intraepithelial cells with irregular nuclear contours (squiggle cells) when the cells become deformed as they migrate between adjacent squamous cells (Cucchiara et al. 1995). In a study by Mangano et al. (1992), biopsies showing evidence of esophagitis of diverse etiologies had an average of greater than six squiggle cells per high-magnification microscopic field, but fewer cells were frequently observed in otherwise normal esophageal biopsies. Similar numbers (mean count of 5 ± 4 lymphocytes per high-magnification microscopic field) were observed in normal control subjects by Haque and Genta (2012). When counting intraepithelial lymphocytes, the pathologist should bear in mind that the number of lymphocytes can vary considerably from one field to another, and therefore the average number may not reflect the severity of changes in the entire biopsy (Rubio et al. 2006). Eosinophils, even as few as one to two in several high-magnification microscopic fields, are considered abnormal in the pediatric population by many investigators (Winter et al. 1982; Dahms 1997), while others consider rare eosinophils to be a normal finding (Goldman 1996). In addition, some of the histologic findings that are diagnostic of esophagitis in the more proximal esophagus are considered normal in the distal esophagus, immediately proximal to the lower esophageal sphincter (Dahms 1997). In general, however, these mild histologic abnormalities in the distal 1–2 cm of the esophagus are more prevalent in adults than in children (Orenstein 1999).
The diagnostic changes of esophagitis can be focal, and multiple biopsies should be obtained in order to minimize sampling error.
2.2 Upper Endoscopy
For pediatric gastroenterologists, esophagogastroduodenoscopy (EGD) is the most useful test in determining the etiology of most esophageal disorders. Although barium radiography and manometric studies are important for the diagnosis of structural disorders and motility abnormalities, respectively, EGD provides a direct method of visualizing abnormal tissue and, more importantly, provides the means to obtain tissue samples for histologic evaluation. Endoscopes are produced in various sizes from 5.4 mm in diameter to 13 mm in diameter and approximately 120 cm in length. Greater diameter endoscopes allow larger biopsy forceps to be passed (larger biopsies). In infants, the distance from the teeth to the gastroesophageal junction is 20 cm, while in adults this distance is 40 cm. It is the responsibility of the endoscopist to choose the correct scope to fit the patient.
EGD should be considered in any child with prolonged upper gastrointestinal symptoms or the prolonged use of medication to treat upper gastrointestinal symptoms. Currently, in most pediatric specialty centers, EGD is performed either in conjunction with an anesthesiologist (propofol or intubation) or via intravenous sedation while the patient is semiconscious. During the procedure not only is visual inspection of the esophagus, stomach, duodenal bulb, and duodenum (past the ampulla of Vater) conducted but also biopsy samples of each area are obtained. While it is essential to collect tissue samples from obvious visual lesions, it is also imperative to collect biopsies when no abnormalities are visualized, as several studies (Black et al. 1988) have demonstrated that simple observation of perceived “normal” tissue may miss significant mucosal abnormalities. Pediatric gastroenterologists routinely collect biopsies from the distal esophagus (2–4 cm above the gastroesophageal junction or the Z-line), the gastric antrum, and the second to third portion of the duodenum.
Biopsies are obtained by using grasp forceps. The most significant problem of EGD is the difficulty in acquiring large enough tissue samples for adequate evaluation by the pathologist. This is especially true when EGD is performed in infants and toddlers as the size of the endoscope and biopsy forceps are limited secondary to the size of the child. In order to circumvent this problem, pediatric gastroenterologists attempt to collect at least two to three specimens from each site. The specimens can be placed directly on specially manufactured gauze in an attempt to orient them; however, in our experience in the process of handling the specimens, crush artifact can occur. Presently, most endoscopy technicians simply shake the biopsy from the forceps directly into formalin, which minimizes the possibility of crush artifact and allows the pathology department to properly orient and prepare the specimens.
Major complications of upper endoscopy, such as perforation, bleeding, pneumothorax, and death are extraordinarily rare. Minor events can occur and include nausea, vomiting or dizziness, pain from an intravenous lines, and a sore throat (Ament and Christie 1977).
2.3 Esophagitis Due to Gastroesophageal Reflux
2.3.1 Clinical Aspects
Gastroesophageal reflux is one of the most common esophageal disorders in children (Boyle 1989). GER is frequently seen in infants but can also occur in children and adolescents. An increased frequency or duration of these episodes may result in pathologic effects on the esophageal mucosa (Fig. 2.1a) or the airways (gastroesophageal reflux disease or GERD). GERD has different clinical manifestations in infants and children (Orenstein 1999). While the main manifestations in infants include nonspecific irritability, apnea, or malnutrition due to regurgitation, older children have similar symptoms to adults (heartburn, regurgitation, epigastric abdominal pain, dysphagia, and occult bleeding). GERD tends to persist in older children, while it usually resolves during the first 1 or 2 years of life in infants. GERD can occur secondarily to other disorders that affect the esophagus such as anatomic abnormalities, food allergy, repaired esophageal atresia, and gastrointestinal dysmotility. A particularly problematic group of patients are those who initially are asymptomatic but present late in the course of the disease when complications of esophagitis develop.
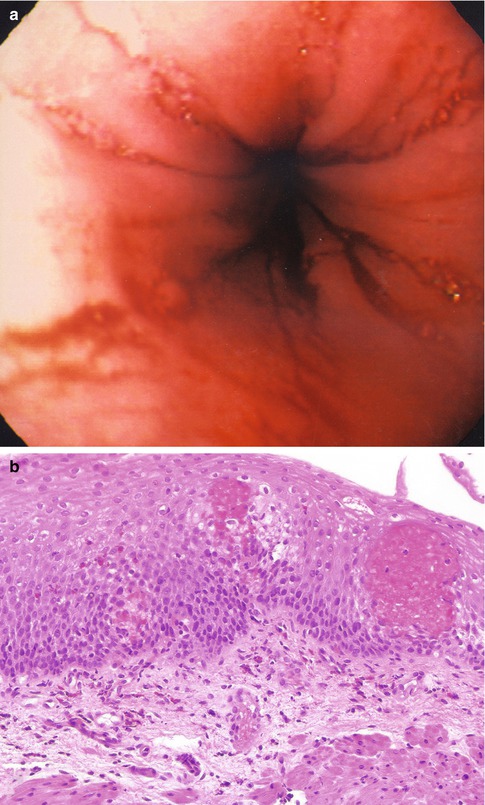

Fig. 2.1
Gastroesophageal reflux. (a) An endoscopic view of an inflamed distal esophagus demonstrating tissue erythema and friability of the mucosa. (b) The biopsy demonstrates basal cell hyperplasia, elongation of the papillae, and a mild eosinophilic infiltrate. As illustrated in this case, the severity of the endoscopic changes do not always correlate with the severity of the microscopic findings
2.3.2 Histology
The histologic findings of GERD encompass three types of changes: intraepithelial inflammatory cell infiltrates, epithelial alterations, and changes in the lamina propria (Table 2.2, Fig. 2.1b). None of these changes is absolutely specific for reflux esophagitis, and they can be seen in other types of esophagitis as well. Intraepithelial eosinophils have been considered as the single most specific diagnostic feature of reflux esophagitis (Winter et al. 1982). However, eosinophils can also be present in conditions such as Crohn’s disease and infections and can be particularly prominent in eosinophilic esophagitis or other gastrointestinal diseases that cause tissue eosinophilia, as discussed in the next section of this chapter. In cases of nonerosive esophagitis, the degree of inflammation in GERD is usually mild, and the number of eosinophils is usually small (Ashorn et al. 2002). Therefore, several biopsy tissue samples may be necessary to establish the histologic diagnosis of esophagitis. In fact, it is not unusual that even in the presence of severe inflammation not all tissue fragments are equally involved and some may show no pathologic changes. Less commonly, a large number of esophageal eosinophils may respond to proton pump inhibitor therapy (Ngo et al. 2006); thus, a diagnosis of GERD or eosinophilic esophagitis cannot simply be made by the pathologist utilizing histologic findings. Often a clinicopathologic diagnosis is required.
Table 2.2
Histologic changes of reflux esophagitis
Epithelial alterations |
Basal cell hyperplasia |
Basal cell spongiosis |
Nuclear enlargement, anisocytosis, and increased mitotic rate in basal layer |
Balloon cells |
Intraepithelial inflammation |
Eosinophils |
Lymphocytes |
Neutrophils |
Lamina propria |
Elongation and increased numbers of papillae |
Vascular dilatation of papillae |
Intraepithelial squiggle cells and round lymphocytes, normal components of the squamous mucosa, are often numerous in reflux esophagitis, but the normal upper limit of these cells is not precisely defined (Mangano et al. 1992; Cucchiara et al. 1995). Neutrophils are absent in the normal esophagus and when present, they almost always indicate a pathologic process. Neutrophils tend to be present less often in reflux esophagitis and are usually more prominent in infectious esophagitis or when ulceration is present.
Accelerated turnover and shedding of epithelial cells may result in basal cell hyperplasia and lengthening of the papillae. In our experience, it is unusual to observe these features when inflammatory cell infiltration is entirely absent, but in a few cases these may be the only changes. Establishing the diagnosis of GERD in this setting may be equivocal, particularly when evaluating small biopsies that are difficult to orient.
It has been suggested that some cases of GERD may have a genetic basis (Ghoshal and Chourasia 2011). Familial segregation has been identified in instances of hiatal hernia, Barrett’s esophagus, esophageal adenocarcinoma, and GERD (Orenstein et al. 2002). Hu et al. (2000) identified a locus in 13q 14 linked to the GERD phenotype. However, Orenstein et al. were not able to replicate this finding and speculated that the discrepancy could be the result of the phenotypic disparity between the subjects of the two studies, in genetic heterogeneity of GERD itself, or both (Orenstein et al. 2002).
2.3.3 Complications
The most important complications of reflux esophagitis are ulceration, peptic strictures, secondary infections, and Barrett’s esophagus. Ulcers secondary to long-standing reflux are nonspecific, but since they may develop secondary infections, evaluation of such a biopsy requires the exclusion of fungi or viruses.
2.3.4 Treatment and Follow-up
The treatment of GERD consists primarily of acid suppression and enhancing gastric emptying. Acid blockade with antacids, H2 receptor antagonists, or proton pump inhibitors are the mainstay of GERD therapy (Karjoo and Kane 1995). While esophageal histology is extremely important in identifying reflux disease, follow-up biopsies are only indicated in those patients who have either erosive esophagitis or long-standing symptoms.
2.4 Barrett’s Esophagus
2.4.1 Clinical Aspects
While Barrett’s esophagus (BE) is a well-known occurrence in adults, it rarely occurs in children. The lower prevalence may be explained by the need to have a prolonged exposure to severe gastroesophageal reflux for BE to develop (Hassall 1997); however, to our knowledge, the actual prevalence of BE in children is unknown. Most cases of pediatric BE are seen in the second decade, although it has been reported as early as 1 year of life (Qualman et al. 1990; Beddow et al. 1999). Some associated conditions such as severe mental retardation, cystic fibrosis, esophageal atresia, and malignancies treated with chemotherapy increase the risk of developing BE (Hassall 1997).
2.4.2 Histology
Although there is an increasing consensus that the diagnosis of BE should be made definitively only when Barrett’s specialized epithelium is present, that is, epithelium containing goblet cells (Sampliner 2002), some pathologists have considered the presence of fundic-type and cardiac-type gastric mucosa 2 cm or more proximal to the lower esophageal sphincter as other types of BE (Dahms and Rothstein 1984). Frequently one sees a mixture of cell types resembling gastric and/or intestinal mucosa which are usually inflamed and lack the organization of their normal counterparts (Fig. 2.2). In a review by Hassal, only 43 cases with Barrett’s specialized metaplasia were identified among 13 reports spanning from 1984 to 1996, in which 119 children were reported to have BE (Hassall 1997). A major difficulty is the interpretation of an esophageal biopsy that includes gastric-type mucosa when proper documentation of anatomic landmarks at the biopsy site is lacking, in that a hiatal hernia or normal gastric mucosa extending more proximally than usual in a zigzag Z-line cannot be ruled out. This issue is further complicated by the controversy that surrounds the significance of cardiac-type mucosa at the gastroesophageal junction, which is discussed below.
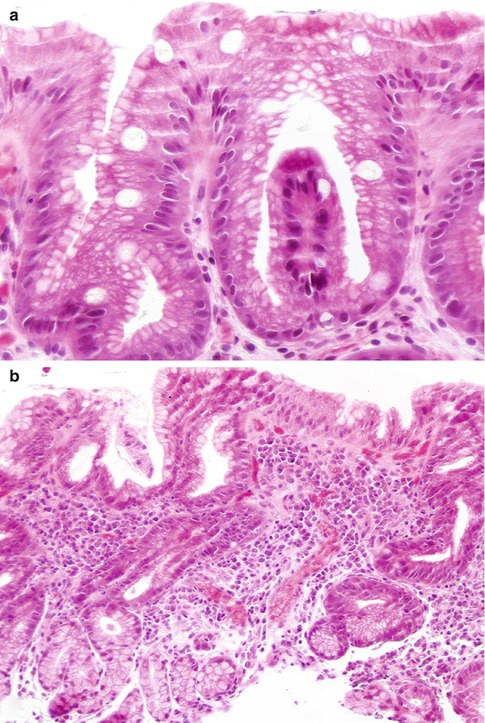

Fig. 2.2
Barrett’s esophagus. (a) Specialized columnar metaplastic epithelium including goblet cells alternates with columnar mucous cells similar to those seen in the gastric foveolar epithelium. (b) Distal esophageal biopsy with inflamed cardia-like mucosa. Goblet cells are absent. Unless there is precise knowledge of the site of the biopsy and a hiatal hernia has been ruled out, it is difficult to be certain whether this type of mucosa represents glandular metaplasia of the distal esophagus or it represents acquired changes in the proximal stomach
Additional controversial criteria include the presence of epithelial cells positive with alcian blue stain at pH 2.5. The use of this stain has been advocated for the identification of acidic mucins, which according to several studies are normally seen only in the intestine but not in the stomach (Lee 1984). However, it has been our experience, and that of others too (Ellison et al. 1996), that alcian blue positive cells can be observed in the gastroesophageal junction of normal fetuses and young children, in the absence of goblet cells (see Fig. 3.1). Furthermore, it is now recognized that hyperdistended gastric foveolar cells (pseudogoblet cells) stain positively with alcian blue and can be mistaken for true goblet cells (Fenoglio-Preiser 2008; Odze and Goldblum 2009). Therefore, reliance on mucin stains to identify metaplastic columnar epithelium, in the absence of goblet cells, may result in overdiagnosis of BE.
The controversial aspects of the gastroesophageal junction are not limited to BE. It has been proposed that the anatomic cardia region may be exclusively comprised of pure oxyntic-type epithelium at birth, while mucous-type glands develop as a metaplastic event and are an early histologic manifestation of GERD (Chandrasoma et al. 2000a). Studies conducted to evaluate the histologic features of the gastroesophageal junction in normal fetuses and children (Kilgore et al. 2000; Zhou et al. 2001; Glickman et al. 2002) and a summary of the literature reported by Odze (2005) suggest that the normal cardia is probably much shorter than traditionally thought (only a few millimeters). However, purely mucous glands or mucous glands admixed with rare parietal cells have been identified at the normal gastroesophageal junction samples from the fetuses and children who were evaluated in these studies. Even if one considers purely mucous glandular epithelium a normal gastric component at the gastroesophageal junction, it is still possible that the mucosa of the lower esophagus may undergo metaplasia into cardia-like mucosa; this change could then result into the “lengthening” of the cardia that is described by some authors (Chandrasoma et al. 2000b; Odze 2005).
2.4.3 Complications
Adenocarcinoma in children with BE is extremely rare, but it has been reported in those as young as 8 years old (Gangopadhyay et al. 1997).
2.4.4 Treatment and Follow-up
Patients with BE need to undergo routine, frequent monitoring. Currently, patients with BE require aggressive acid suppression. In addition, while many physicians advocate antireflux surgery in order to minimize gastroesophageal reflux, these measures do not guarantee resolution of the disease, particularly in children (Beddow et al. 1999)
2.5 Eosinophilic Esophagitis (EoE)
2.5.1 Clinical Aspects
Over the last two decades, it has been recognized that not all cases that present with symptoms of GER and esophageal eosinophilia are secondary to gastroesophageal reflux disease (Kelly et al. 1995). Since 2003, a number of studies have described a new, unique group of patients with a severe esophageal eosinophilia who present with symptoms that are otherwise indistinguishable from those secondary to reflux esophagitis but fail to respond to conventional antireflux therapy or antireflux surgery (Liacouras and Markowitz 1999; Walsh et al. 1999; Orenstein et al. 2000; Furuta 2001, 2002). A recent set of guidelines in 2011 were developed for “eosinophilic esophagitis” and include the following: (1) patients present with and isolated esophageal eosinophilia (other GI pathology is normal) and symptoms of esophageal dysfunction; (2) the degree of eosinophilia in these patients is almost always greater than 15 eosinophils per high-magnification microscopic field; (3) disorders such as GERD and PPI-responsive esophageal eosinophilia be excluded; (4) and the symptoms and histologic abnormalities improve with either steroid or restriction diet therapy (Liacouras et al. 2011). It is important to make the distinction between esophageal eosinophilia, a pathologic description, and eosinophilic esophagitis, a clinicopathologic disease (Table 2.3).
Table 2.3
Causes of esophageal eosinophilia
Eosinophilic esophagitis |
Gastroesophageal reflux disease |
PPI-responsive esophageal eosinophilia |
Eosinophilic gastroenteritis |
Crohn’s disease |
Hypereosinophilic syndrome |
Achalasia |
Vasculitis, pemphigus, and connective tissue disease |
Infection |
On the basis of these observations and the frequent association of extraintestinal allergic symptoms (asthma, eczema, and chronic rhinitis), investigators have realized that these patients have an antigen (food)-/immune-mediated etiology for this type of esophagitis. While esophageal histology is essential in making the diagnosis of EoE, in some cases, endoscopy may reveal either a “ringed” appearance or linear “furrows”; however, just like the histopathology, visual endoscopic features are not pathognomonic for the disease (Fig. 2.3a, b). Liacouras et al. (1998) demonstrated that the clinical and histologic features of EoE might evolve over years. Some patients may actually remain asymptomatic and present late in the course of the disease with dysphagia and food impaction (Orenstein et al. 2000; Furuta and Straumann 2006). The main features of EoE are listed in Table 2.4 (Liacouras and Markowitz 1999).
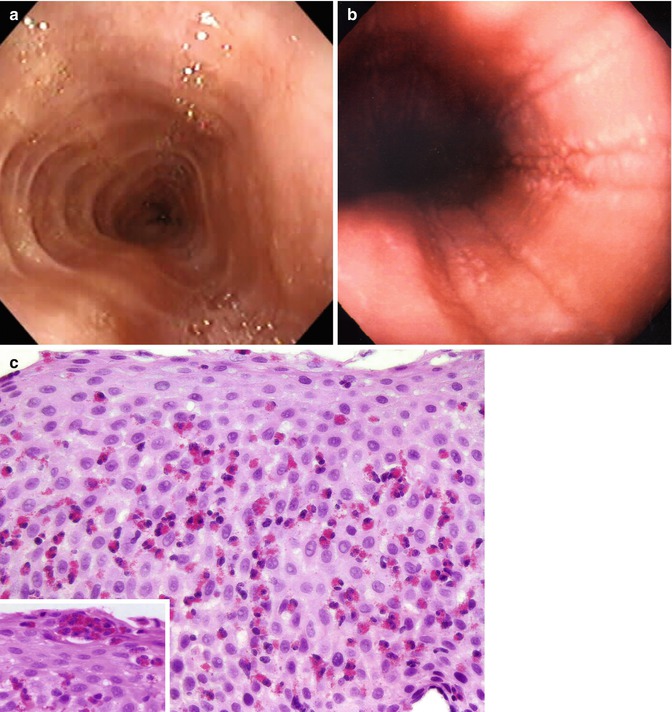

Fig. 2.3
Eosinophilic esophagitis. Notice in the endoscopy the ringed appearance (felinization) of the esophagus (a) and multiple deep linear furrows (b). The biopsy (c) demonstrates massive eosinophilic infiltrate with clusters of eosinophils near the epithelial surface (inset)
Table 2.4
Eosinophilic esophagitis
Gastrointestinal symptoms | Vomiting/regurgitation |
Epigastric and chest pain | |
Heartburn | |
Nausea | |
Dysphagia | |
Extraintestinal symptoms | Asthma |
Eczema | |
Chronic rhinitis | |
24-h pH probe testing | Normal |
Histology | Severe esophageal eosinophilia |
Treatment | Unresponsive to antireflux therapy |
Responsive to steroids and elimination diet |
2.5.2 Histology
In patients with EoE, the histologic changes are similar to reflux esophagitis, but the number of intraepithelial eosinophils is usually higher, frequently greater than 15 eosinophils per high-magnification microscopic fields (Ruchelli et al. 1999; Walsh et al. 1999; Orenstein et al. 2000) (Fig. 2.3c). No prospective studies have determined a threshold number of esophageal eosinophils that can establish a diagnosis of EoE with high specificity and sensitivity. EoE should be diagnosed by clinicians, taking into consideration all clinical and pathologic information; neither of these parameters should be interpreted in isolation. In addition to the eosinophil-predominant inflammation, other histologic features present in cases of EoE include eosinophilic aggregates or microabscesses, surface layering of eosinophils, numerous eosinophils mixed with desquamated luminal debris, extracellular eosinophil granules, basal cell hyperplasia, and lamina propria fibrosis (Figs. 2.3 and 2.4). Although some of these features may favor EoE, they are not considered perfect for discriminating EoE from GERD. Given the frequent patchy distribution of these findings, multiple biopsy specimens from the proximal and distal esophagus should be obtained; however, the vast majority of pediatric studies have shown that the distal esophagus is almost always more involved than the proximal esophagus. A few studies have shown that significant eosinophilic inflammation occurs in the proximal esophagus of adults with EoE but not GERD (Lee et al. 2010); others have not confirmed this finding (Molina-Infante et al. 2011), and recent evidence suggests that adults with EoE also have primarily distal esophageal involvement (Lucendo et al. 2013). When eosinophilic infiltration is not confined to the esophagus but involves other segments of the gastrointestinal tract, classic eosinophilic gastroenteritis should be considered. Whether EoE is a variant of eosinophilic gastroenteritis or represents a different process is not clear (Goldman and Proujansky 1986; Liacouras and Markowitz 1999; Kelly 2000); however, most current investigators believe that EoE is a distinct disease process.
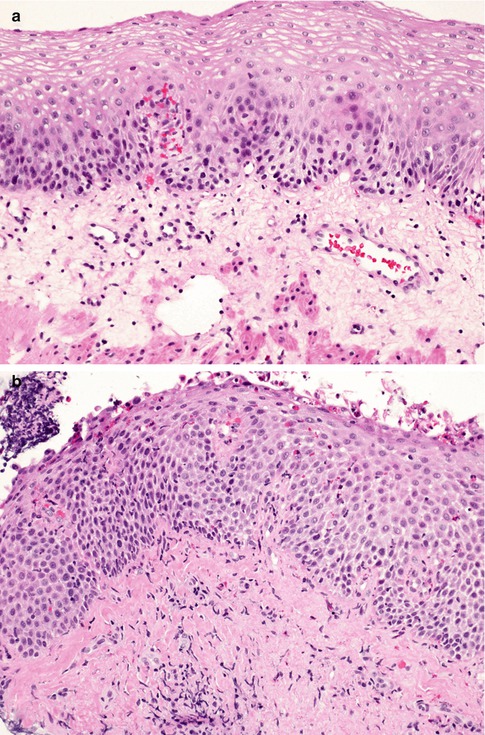

Fig. 2.4
Eosinophilic esophagitis. Compare the loose texture of the connective tissue of the lamina propria in the normal esophagus (a) with the dense connective tissue of the fibrotic lamina propria in this case of eosinophilic esophagitis (b)
2.5.3 Complications
Because EoE has only recently been recognized, both acute and long-term complications have been difficult to assess. A review of the literature demonstrates that EoE occurs in both children and adults (Fox et al. 2002). Esophageal fibrosis in the deeper submucosal and muscular layers occurs in many patients with EoE (Aceves et al. 2007). Adolescents and adults diagnosed with this disorder often suffer from significant dysphagia and the development of esophageal strictures. Left untreated, EoE may cause a severe narrowing of the esophagus.
2.5.4 Treatment and Follow-up
Many reports have demonstrated that the disease responds to corticosteroid therapy, either taken orally or by swallowed inhalation therapy; however, many patients have a high rate of recurrence after discontinuation of therapy (Liacouras et al. 1998). Patients have also been found to respond to either a strict food elimination diet using an elemental formula (Kelly et al. 1995; Furuta 2002) resulting in complete resolution of esophageal eosinophilia and its corresponding symptoms or a variable food restriction diet (Kagalwalla et al. 2006). The current treatment for EoE includes both dietary exclusion, topical swallowed corticosteroids or systemic steroids, or a combination of each (Liacouras et al. 2011).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








