General Aspects of Operative Hysteroscopy
Michael S. Baggish
Hubert Guedj
Rafael F. Valle
The ability to intervene surgically with the hysteroscope has substantial advantages for the patient, the physician, and the third-party payer. Psychologically, an operation that is performed without incisions and is followed by rapid recovery and diminished pain is perceived by the patient with far less apprehension and greater tranquility than more complex procedures. The physician can perform operations in a more expeditious, exact manner within shorter periods of time. The risk of postoperative complications, for example, wound infection, is greatly diminished, and the chance of some postoperative problems, such as wound dehiscence, is reduced to zero. Hysteroscopic operations, in contrast to open laparotomy, translate to shortened hospitalization, reduced operating time, and reduced costs. Additionally, some operative techniques have been made obsolete by the advent of operative hysteroscopy. Virtually no one performs metroplasty by cutting open the abdomen and incising the uterus; rather, the septum is simply cut by way of the endoscopic approach. A further accrued benefit is that the hysteroscopic approach to treating a uterine septum means that the woman who subsequently conceives can be delivered of the infant by the vaginal route, in contrast to the abdominal metroplasty, which always requires delivery by cesarean section.
Procedure
The choice of the distending medium rests primarily with the individual surgeon. Liquid media have the advantage of being cleared from time to time by aspiration or by flushing. CO2 is not useful when bleeding is encountered. Similarly, there is substantial individual variation and preference in choice of instruments (e.g., rigid, flexible, laser, or electrosurgical). Few experienced hysteroscopic surgeons select flexible instruments because they are simply too fragile and flimsy to perform the job adequately. Not infrequently, the best way to complete a complicated procedure requires the use of more than one instrument (e.g., combination of scissors and an electrode). The experienced surgeon will have developed familiarity and skill with a more-than-one-instrument system. Clearly, compulsive preoperative preparations may reflect whether the hysteroscopic procedure is easy or difficult, successful or unsuccessful, skilled or amateurish.
Preparation
Every patient deserves and requires a complete preoperative evaluation starting with a history and physical assessment. Particular items of interest include the following: character, timing, and amount of uterine bleeding; tendency to bleed from extragenital sites; cardiac abnormality (e.g., prolapsed mitral valve, history of rheumatic fever, septal defects); drug therapy relative to possible interactions; and extragenital endocrine disorders. During the physical examination, attention should be focused on vaginal access to the cervix; the status of the cervical canal; the size, consistency, and position of the uterus; the size of the adnexa; and the status of the rectum and bladder. The presence of local infections of the vagina and cervix should be noted and treated before hysteroscopic surgery. Appropriate laboratory studies and consultations should be arranged. If indicated, hysterograms should be obtained and personally viewed by the surgeon. The authors prefer to perform office hysteroscopy, if this is agreeable to the patient, to further supplement the diagnostic survey. When the workup is complete, every woman should be given the opportunity for thorough, informed consent, including a description of possible side effects and complications of operative hysteroscopy. Drawings and notations of the explanation should be documented in the record. The patient should know in detail what she is giving permission to have performed.
Concurrent Laparoscopy
When simultaneous laparoscopy is required or desired, the patient should be informed about the abdominal incisions needed for laparoscopy and should be prepared for possible complications associated with this aspect of the procedure. Likewise, when the operation is posted with the operating room, a list of instruments and accessories for hysteroscopy should be requested; this should include appropriate energy devices, fibers, and electrodes. Suitable time should be allocated for combined procedures. When there is a risk of bleeding (e.g., myomectomy, takedown of extensive adhesions), the patient should have a blood sample drawn for type and hold. Obviously any woman with rheumatic heart disease, prolapsed mitral valve, or any other valid indication should be covered by appropriate antibiotic prophylaxis for genitourinary surgery.
Preparation of the Endometrium
Frequently the timing of the operation determines the success or failure of the hysteroscopic surgery. We prefer to treat our patients with gonadotropin-releasing hormone (GnRH) agonist preoperatively to render the endometrium atrophic prior to major hysteroscopic surgery unless these drugs are contraindicated. An alternative to preparation with a drug is to plan the surgery for the early proliferative phase of the cycle. Surgery during the secretory phase is more difficult because vision is suboptimal and also because bleeding is more likely to eventuate.
Cervical Softening
Several studies have evaluated cervical softening or dilatory agents to allow either no dilatation or easier dilatation during hysteroscopy. One study (Darwisham et al., 2004) compared the actions of misoprostol and laminaria and found them equally effecting in allowing easier dilatation. Fernandez et al. (2004) found that vaginal misoprostol applied 4 hours prior to operative hysteroscopy did not reduce the need for cervical dilatation and did not render the hysteroscopic surgery easier. Ben-Chetrit et al. (2004) found that mifepristone did not create cervical softening in preoperative hysteroscopy patients.
Video Hysteroscopy
Depending on the skill of the surgeon, the operation may be performed under direct vision or indirectly by attaching a microchip television camera to the eyepiece of the endoscope and operating by viewing the video monitor. The latter offers so many advantages (e.g., allowing the assistants and nursing staff to fully observe the operation, as well as permitting the surgeon to sit upright and view his or her instruments) that it has virtually replaced direct-view surgery. This technique, additionally, is a convenience to the laparoscopist when combined operations are done. The major disadvantage of video hysteroscopy relates to the two-dimensional view afforded to the surgeon. Furthermore, a clear video image is required to operate from the monitor. The picture should be sharp, and the quality of the color, brightness, and contrast should be the equivalent of commercial television (Fig. 24.1).
For prolonged procedures, it is advantageous to always request the circulating nurse to accurately record and read out the quantity of liquid medium used and the volume recovered (returned). Additionally, it is advantageous to inform the anesthesiologist about side effects of the selected medium prior to surgery.
Positioning
Positioning of the patient is of prime importance, as is accurate determination of the position of the uterus. The buttocks should be positioned so that they are supported by the operating table and not hanging off the table. The inferior extremities should not be hung in the so-called high lithotomy position. They should be gently flexed in a medium lithotomy position and slightly abducted. Care should be taken not to trap the external iliac vessels beneath the inguinal
ligaments. Additionally, the knees should not be compressed against the leg supports. The principal nerves susceptible to injury are the sciatic (peroneal division at the knee, main trunk at buttocks) and femoral (flexion, abduction positions) nerves. Proper positioning of the operator relative to the patient’s uterus completes this important phase of the preparation for surgery. The authors suggest having an operating stool equipped with wheels to allow easy shifting of the surgeon’s position. Ideally, a hydraulic up-and-down control will allow even greater facility when the operator desires to change his or her position.
ligaments. Additionally, the knees should not be compressed against the leg supports. The principal nerves susceptible to injury are the sciatic (peroneal division at the knee, main trunk at buttocks) and femoral (flexion, abduction positions) nerves. Proper positioning of the operator relative to the patient’s uterus completes this important phase of the preparation for surgery. The authors suggest having an operating stool equipped with wheels to allow easy shifting of the surgeon’s position. Ideally, a hydraulic up-and-down control will allow even greater facility when the operator desires to change his or her position.
Reconnaissance
Initially, before the surgeon inserts the operating hysteroscopic system, a scout diagnostic examination of the uterus should be done. This rapid-scanning endoscopy gives the surgeon a final view of the uterine cavity prior to initiating the operative portion of the procedure and affords the advantage of last-minute adjustments to the surgical plan.
Experienced endoscopists check all operating instruments to be sure they work before beginning surgery.
Maintaining Uterine Distension
Perhaps the greatest difficulty insofar as the technical aspects of operative hysteroscopy are concerned is the inability to maintain uterine distension. This problem is principally encountered when the cervix is overdilated. Although the correction is not perfect, this situation may be remedied by the strategic placement of a purse-string suture just below the bladder reflection. Excessive leakage may be prevented by due care when dilating the cervix. Distension may also be lost if there is a faulty seal covering the operating channel.
Choice of Anesthesia
Operative hysteroscopy for minor problems may be performed under local anesthesia because these procedures usually last 30 minutes or less. This type of anesthesia may be administered by paracervical block or, preferably, by injection of the local anesthetic directly into the substance of the cervix. Several agents are available, but the simplest drug that provides adequate anesthesia for short procedures is 1% Xylocaine without additives. Alternatively, chloroprocaine 1% (Nesacaine) is an excellent choice. For longer operations, Marcaine may prove to be advantageous. Lau et al. (2000) performed a randomized, blinded, placebo-controlled evaluation of 100 women undergoing hysteroscopy comparing paracervical block using 10 mL lignocaine versus normal saline. The anesthesia group had pain reduced only at the time of hysteroscope insertion but not during the remainder of the operation. Additionally, the anesthesia group had a higher incidence of bradycardia and hypotension. In another study, Lau et al. (2000) instilled local anesthesia into the uterine cavity to obtain anesthesia. The local anesthesia failed to relieve pain and did not prevent vasovagal reactions. Fewer than 5% of our cases have been done under regional blockade. This has not been a popular anesthetic technique for either physician or patient. General anesthesia has proven to be the safest and most popular method of anesthesia for operative endoscopy. Even high-risk patients may enjoy the safest of cardiac general anesthesia. General anesthesia is beneficial when the patient is expected to be in the uncomfortable lithotomy position for more than 30 minutes.
Mushambi and Williamson (2002) emphasize the potential for serious complications that can occur during and after operative hysteroscopy. They point out that anesthesiologists often relate these complications to similar problems that occur during transurethral prostatectomy. However, complications such as fluid overload, hyponatremia, hypo-osmolality, hemorrhage, uterine perforation, and gas or air embolism are not entirely comparable to hysteroscopic surgery. Hyponatremia and hypo-osmolality occurred in up to 6% of cases. Hong et al. (2002) compared sevoflurane-nitrous oxide and target-controlled propofol with fentanyl anesthesia for hysteroscopy via a randomized prospective
study. They concluded that sevoflurane-nitrous oxide was superior for day-case hysteroscopic surgery relative to speed on anesthetic recovery (316 vs. 507 seconds) and return to hemodynamic stability (380 vs. 666 seconds).
study. They concluded that sevoflurane-nitrous oxide was superior for day-case hysteroscopic surgery relative to speed on anesthetic recovery (316 vs. 507 seconds) and return to hemodynamic stability (380 vs. 666 seconds).
Procedures
Directed Hysteroscopic Biopsy
There is very little logic in modern obstetrics and gynecology for the performance of a blind dilatation and curettage. The procedure of choice to investigate potential or probable intrauterine disease is hysteroscopy combined with either biopsy sampling or directed curettage. Roberts et al. demonstrated that vigorous curetting of endometrial carcinoma led to the appearance of malignant cells in inferior vena cava blood samples; therefore, selective sampling would appear to be safer for the patient at risk.
In contrast to the aforesaid, Ben-Yehuda et al. in a retrospective study determined whether hysteroscopy improved the diagnostic sensitivity of dilatation and curettage based on impressions of cancer or hyperplasia. The authors concluded that hysteroscopy did not improve the sensitivity of dilation and curettage. However, Clark et al. recently published a review article on the subject of hysteroscopic accuracy in the diagnosis of endometrial cancer and hyperplasia in the Journal of the American Medical Association (2002) based on 208 eligible articles and 65 primary articles encompassing 26,346 women. They reported that a positive hysteroscopy result increased the probability of cancer from 3.9% to 71.8% whereas a negative hysteroscopy reduced the probability of cancer to 0.6%. The authors concluded that the diagnostic accuracy of hysteroscopy is high for endometrial cancer. Bain et al. (2002) reported that outpatient hysteroscopy was as acceptable as an outpatient endometrial biopsy and was most useful in selected cases.
Hysteroscopy used in a fashion analogous to colposcopy with the focus being directed biopsy clearly makes the most sense. The advantage of accurate sampling aided by direct vision versus blind, i.e., chance sampling, is the issue that should be prospectively addressed. Obviously, the endoscopist should be well trained and skilled. An accrued advantage of hysteroscopic examination versus blind sampling is the ability to recognize additional gross disease within the uterus, e.g., polyps, myomas, foreign bodies.
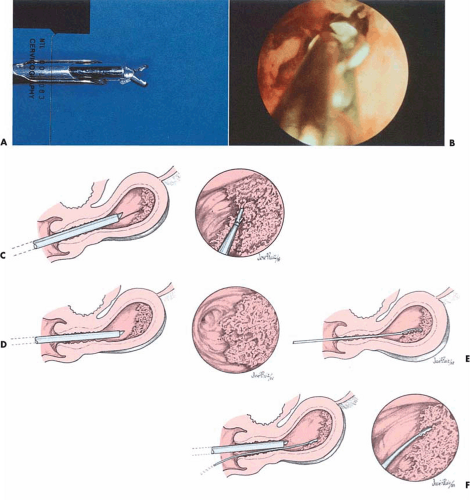
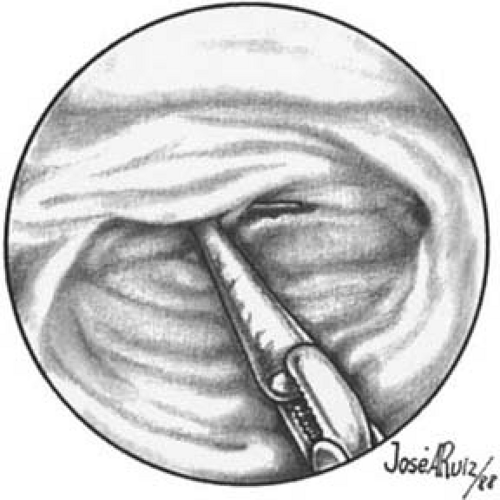
Direct hysteroscopic biopsies are carried out with either a flexible or semirigid biopsy forceps. The major disadvantage of the instrumentation relates to the small volume of tissue obtained (Fig. 24.2). The jaws of a semirigid biopsy forceps, for example, hold a total volume of 0.1 cc. Even under ideal circumstances an endometrial section may be difficult for the pathologist to interpret, and when faced with a minuscule sample the chance for error in diagnosis will be amplified. A 3-mm biopsy forceps obtains approximately twice the volume of tissue as the smaller instruments, and if more than one piece of tissue is secured, the chance for accurate diagnosis increases. An alternative and acceptable technique uses the hysteroscope to identify the lesion to establish a presumptive diagnosis as well as to localize the site of the disease process. The endoscope is then withdrawn, a curette is directed to the known location of the lesion, and a plentiful sample of tissue is removed and sent to the pathologist for diagnosis. Still another method retains the hysteroscope in place but relies on the endoscopist to carefully insert a small curette into the cervix and uterine cavity close to the hysteroscope and then obtain a directed sample. A similar technique may be accomplished with an isolated channel operating sheath but substituting a 3-mm hollow, plastic cannula with suction under direct vision (Fig. 24.3). The latter technique is analogous to that used for chorionic biopsy (Fig. 24.4).
Removal of Intrauterine Foreign Bodies
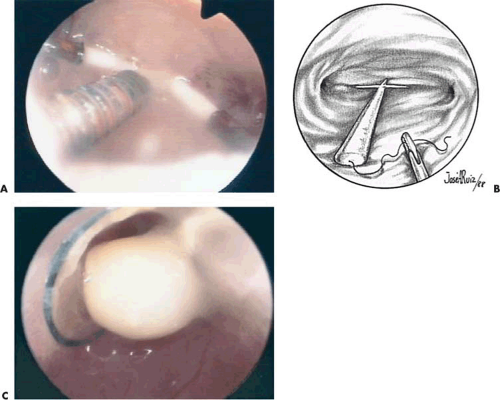
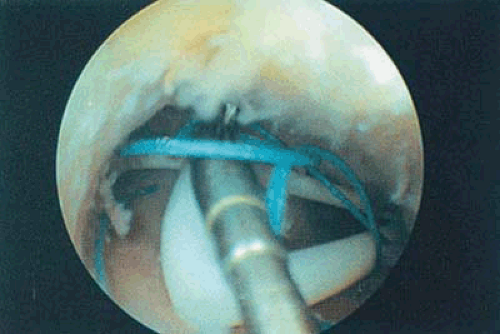
The best method to remove “lost” intrauterine devices (IUDs) lying in the endometrial cavity is under direct hysteroscopic view. Not only can one see where the device is located, but this precise observation also eliminates the trauma of blind probing with resultant bleeding and increased potential for infection (Fig. 24.5A–F). Several methods have been used to extract the IUD. Using a flexible or semirigid grasping instrument and maintaining the hysteroscope just above the internal cervical os provides a panoramic view of the cavity. The IUD string is sighted and seized, and the hysteroscope is withdrawn, dragging the IUD with it through the cervix (Figs. 24.6A, B, 24.7, 24.8).
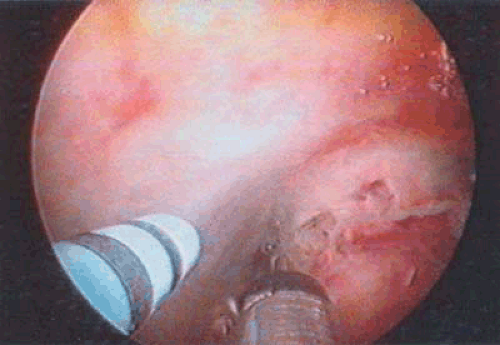 FIGURE 24.3 A 3-mm cannula is placed within the uterine cavity. By applying suction, a specimen may be sucked into the cannula under direct vision and sent for evaluation in the pathology laboratory. |
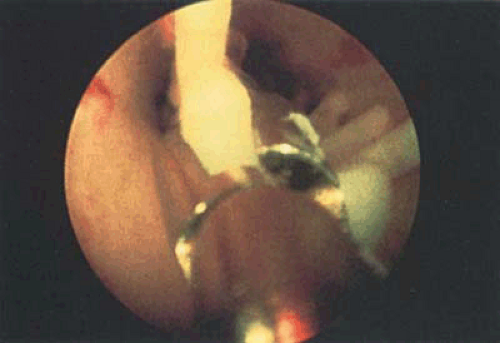
Another method for IUD removal uses the grasping forceps to grab the stem of the device followed by removal through the uterus to the exterior (Fig. 24.9). For the extraction of embedded or fragmented devices, the instrument of
choice is the powerful 3-mm grasping forceps (Fig. 24.10). A piece of the IUD is securely grasped, and while the surgeon maintains steady pressure, the sheath and endoscope are pulled down through the uterus and cervix. The experienced surgeon will carefully locate a sturdy part of the fractured IUD; once the forceps is locked onto the device, he or she will not release it or attempt to seek a new point to grasp. Invariably, release of the device will be accompanied by bleeding, and it may not be possible to locate it again or lock onto it. For embedded devices, a simultaneous laparoscopy is helpful in determining whether the device has penetrated through the uterine serosa. The latter may require a transabdominal removal. Similarly, if a device is fragmented and must be brought out piecemeal, the logical plan for removal is plotted (e.g., first, removal of the upper portion, followed by the lower portions).
choice is the powerful 3-mm grasping forceps (Fig. 24.10). A piece of the IUD is securely grasped, and while the surgeon maintains steady pressure, the sheath and endoscope are pulled down through the uterus and cervix. The experienced surgeon will carefully locate a sturdy part of the fractured IUD; once the forceps is locked onto the device, he or she will not release it or attempt to seek a new point to grasp. Invariably, release of the device will be accompanied by bleeding, and it may not be possible to locate it again or lock onto it. For embedded devices, a simultaneous laparoscopy is helpful in determining whether the device has penetrated through the uterine serosa. The latter may require a transabdominal removal. Similarly, if a device is fragmented and must be brought out piecemeal, the logical plan for removal is plotted (e.g., first, removal of the upper portion, followed by the lower portions).
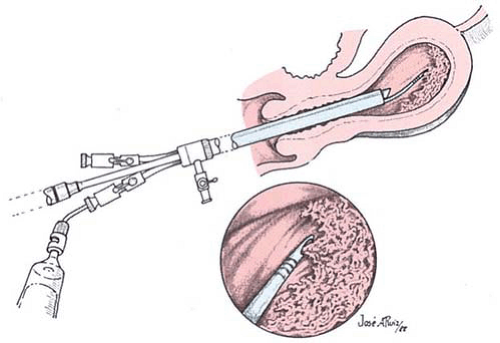 FIGURE 24.4 The hysteroscope locates the diseased tissue, and a sample is suctioned up into plastic tubing inserted either through the operating channel or outside the sheath. |
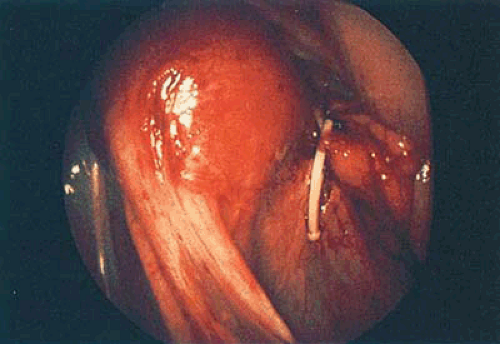
Nonembedded devices may be easily extracted under local anesthesia; however, embedded or fragmented devices require substantial manipulations as well as adjunctive laparoscopy and will be best accomplished under general inhalation anesthesia. Valle and Sciarra reported hysteroscopic IUD extraction in 15 women. Successful removal was carried out in 11; 4 women had empty cavities. Siegler and Kemmann reported ten women who underwent hysteroscopic examination for occult IUDs. Hysteroscopy failed to detect the misplaced devices in two women (one with an embedded device wholly within the myometrium, one whose device was obscured by the amniotic sac). An additional case revealed only a small portion of the device visible by hysteroscopy (i.e., the device perforated the lower uterine segment). In the latter instance, the device was preferentially removed by laparoscopy (Fig. 24.11).
Removal of Endometrial Polyps
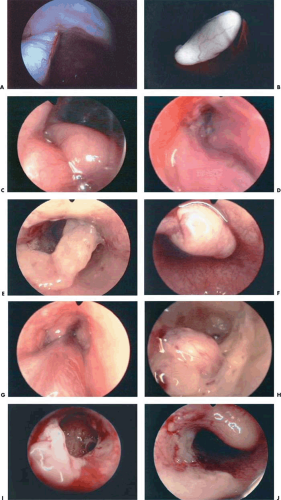
It is well known that uterine polyps are often missed by blind curettage. Valle (1981) reported that polyps were missed in 150 of 179 patients at curettage (Table 24.1). Hysteroscopy is the procedure of choice for locating and treating polyps. It enables one to differentiate between polyps and other space-occupying lesions within the uterine cavity. De Wit et al. (2003) performed 1,045 diagnostic hysteroscopies over a period of 6 years, finding a normal cavity in 54.2% of cases and myomas or polyps in 35% of cases. The authors concluded that hysteroscopic examination was a valuable tool for diagnosing intracavital pathology. Gebauer et al. (2001) performed a prospective study to determine whether hysteroscopy improved the detection and extraction of endometrial polyps. Hysteroscopy identified polyps in 51 cases compared with only 22 by curettage only. The authors concluded that curettage alone in postmenopausal patients is not sufficient for the detection and extraction of endometrial polyps. Additionally, hysteroscopy is the only method by which one can accurately locate the polyp pedicle, with the possible exception of instances where the polyp prolapses through the cervix and is visible to the naked eye or to the magnification of the colposcope (Fig. 24.12A–J). Once the polyp pedicle has been precisely located, operative hysteroscopy may be performed and the pedicle can be cut with flexible scissors, a laser fiber, or an electrosurgical needle electrode. Attention should be drawn to the fact that the polyp(s) is often attached near the fundus, and the approach to the pedicle can be difficult. When the polyp is lying over the posterior wall of the uterus the pedicle is not visible, and the operator may have to push the tip of the endoscope between
the polyp and the posterior wall and lift it up (Fig. 24.13A–I). The same problem may be resolved with scissors (i.e., the operator slips the scissors between the polyp and the posterior wall to swing the polyp upward).
the polyp and the posterior wall and lift it up (Fig. 24.13A–I). The same problem may be resolved with scissors (i.e., the operator slips the scissors between the polyp and the posterior wall to swing the polyp upward).
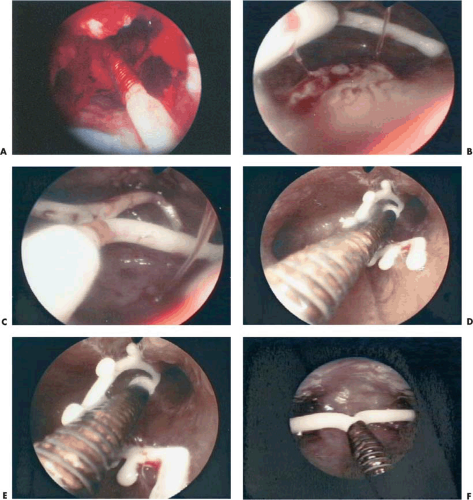 FIGURE 24.5 A: Copper 7 IUD lying in the uterus. B: Displaced Progestasert T IUD and hyperplasia on the posterior wall. The string is visible on the left arm of the device. C: The Progestasert is in the fundus, but filament is ascended. D: IUD is displaced in the left cornua, and the arms are distorted. E: Close-up of Figure 24.5D. F: Copper T device associated with chronic endometritis. The tiny whitish papillary projections are due to local inflammatory reaction. |
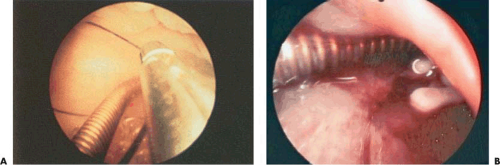 FIGURE 24.6 A: The filament of the IUD is grasped for direct removal. B: “Lost IUD” multiload 375 embedded in thick endometrium. |
TABLE 24.1 Studies of Missed Polyps | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree