Chapter 57 Gastroduodenal and Colonic Endoprostheses
Technical Review
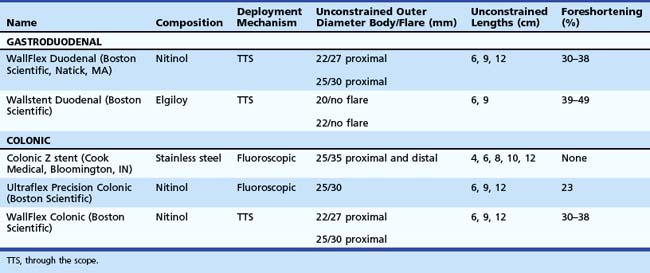
Stent designs and deployment systems are evolving rapidly worldwide, with new devices arriving frequently on the market. Three SEMS for gastroduodenal use and two for colonic obstruction have been approved by the U.S. Food and Drug Administration (FDA) and are available in the United States (Table 57.1). These are all uncovered to reduce the risk of migration. Over time, uncovered SEMS are expected to be incorporated into the surrounding tissues.1 These devices become permanent shortly after placement and require surgical resection for removal once there is significant tissue ingrowth through the mesh. No uncovered SEMS are approved by the FDA for benign disease at this time. Endoscopists should become familiar with several important characteristics of enteral stents, including their composition, lattice pattern and width, flexibility, expandability, deployment mechanism, and degree of foreshortening after placement in the bowel.2
The flexibility and expandability of a stent are directly related to its primary composition.3 Most SEMS are made from shape-memory alloys such as nitinol (nickel-titanium) and Elgiloy (cobalt-chromium-nickel), which balance the need for strong radial forces to expand a malignant stricture while maintaining enough flexibility to minimize the risk of bowel perforation and allow for deployment in tortuous segments of the bowel. SEMS are made in one of two general designs. Stents with large delivery systems that cannot be passed through a therapeutic endoscope are often referred to as over the wire enteral SEMS. These are typically designed for obstructions in the rectum and sigmoid colon and have a larger internal diameter when fully deployed. Most newer models are designed for through the scope (TTS) deployment, in which the stent is contained within a sheath that can be fitted into the working channel of a therapeutic endoscope (3.7-mm).
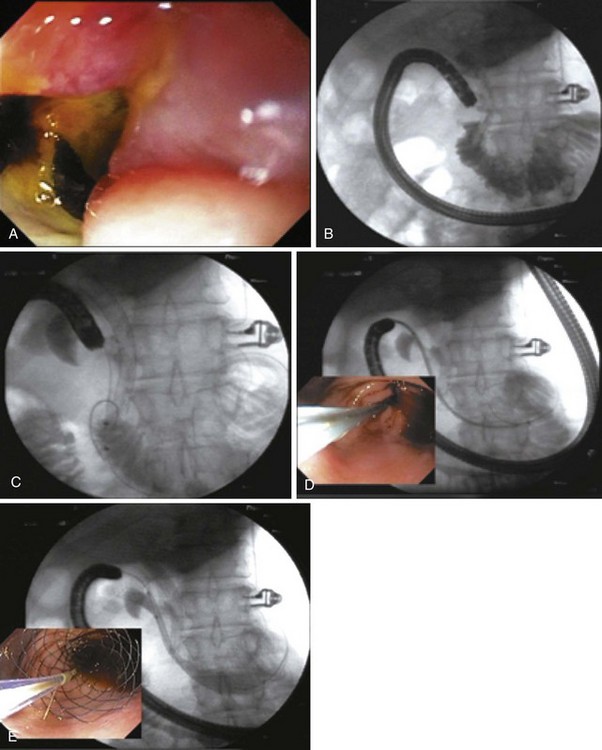
As additional stent designs from various manufacturers become commercially available, it is convenient to describe all stents by their characteristics: stent type (TTS or over the wire), design (woven or laser cut), covering, deployed length and diameter, shortening percentage at deployment, potential for reconstrainability during deployment, and size of the endoscope channel required to accommodate the deployment system. SEMS design allows stent placement under endoscopic and fluoroscopic visualization even in cases where the endoscope cannot traverse the stricture, provided that a guidewire and stent deployment system can be advanced into the correct position (Fig. 57.1). In this setting, a guidewire is advanced across the stricture, which may be facilitated by using a standard endoscopic retrograde cholangiopancreatography (ERCP) catheter or a multilumen biliary extraction balloon catheter. Contrast agent injection and fluoroscopy can be helpful in delineating the anatomy. Once the stenosis is engaged and passed with the guidewire, the balloon catheter can be advanced beyond the stenosis, and the length of the stricture can be measured using the inflated balloon and fluoroscopic image. The guidewire is left in place, and the initial catheter is exchanged for an appropriately sized SEMS system. The stent and sheath mechanism can be advanced over the guidewire without having to remove the endoscope. Once correctly positioned across the stricture, the stent can be released by an assistant as the stent position is carefully maintained by the endoscopist. Several stent deployment systems allow recapturing of the partially deployed stent if the position is suboptimal. Currently available TTS enteral stents continue to expand and foreshorten over several days after being released until the full external diameter is achieved. The endoscopist should account for this in choosing the length and position of the stent before its deployment.
Malignant Gastroduodenal Obstruction
Tumors of the periampullary region, distal stomach, and proximal duodenum often manifest with symptoms of nausea and vomiting secondary to gastric outlet obstruction (GOO). Because most of these patients have unresectable disease, symptom palliation is typically the primary goal. Before the advent of SEMS, surgical gastrojejunostomy had been used to bypass the obstruction. Despite limiting surgical decompression to good surgical candidates, this option would still be associated with significant morbidity and mortality.4
Among patients with GOO, clinical success is typically defined as resolution of symptoms (nausea, vomiting) and tolerance of a soft diet. Comparative trials have shown the superiority of SEMS compared with surgical bypass in palliation of symptoms, length of hospitalization, and cost-effectiveness.5–9 A systematic review of 606 cases of GOO reported technical success and clinical response rates to enteral stent placement of 97% and 87%.10 Newer stent designs such as the WallFlex Enteral stent (Boston Scientific) and the Niti-S Enteral Colonic stent (Taewoong Medical Co., Ltd., Kyonggi-Do, South Korea) are promoted for their greater flexibility while maintaining sufficient radial forces to open strictures.11–13 These stents have blunt woven ends theoretically to reduce the risk of wire penetration and subsequent perforation. As a result of its woven design, the Niti-S stent has less foreshortening (23%) compared with other TTS enteral stents, but the clinical significance of this characteristic is unclear.12 Although there are no comparative trials available, case series using these stents suggest their equivalence to older devices. A multicenter, randomized study comparing the Enteral WallFlex stent with surgical gastrojejunostomy suggested better short-term (<2 months) clinical improvement after stent placement but more favorable long-term results with surgical bypass.14 Although stent placement was associated with lower initial inpatient costs, the differences in long-term costs and incremental cost-effectiveness of either approach are minimal.15 When deciding between endoprosthetic placement and surgery, the patient’s comorbidities and anticipated long-term prognosis are the most important considerations.
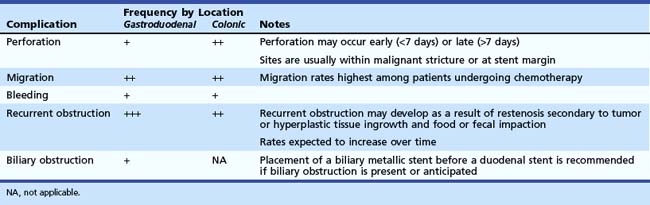
Complications of stent placement for GOO include perforation, bleeding, stent migration, restenosis, biliary obstruction, and failure to expand fully, despite technically successful placement (Table 57.2).10,16 Perforation (0.7%) and bleeding (0.5%) rates are low, but these are potentially life-threatening complications that warrant discussion with the patient. Newer stent designs advertise greater flexibility and blunt ends to reduce the rate of perforation, but there are no data to support these claims.11,12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree