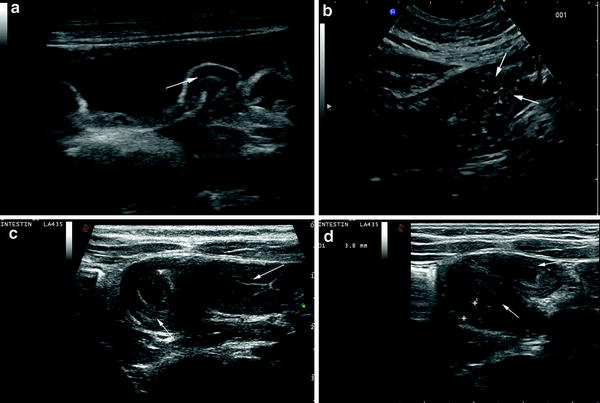
Fig. 1
Newborn infant with cystic fibrosis, presenting with intestinal occlusion due to meconium ileus. Small empty colon (C) (a) is associated with distension of the small-bowel (asterisks) with liquid (arrows) and inspissated faecal content (b, c)
Involvement of the appendix may be found in 16 % of patients with CF, but acute appendicitis is uncommon, affecting 1–2 % of CF patients compared with an overall 7 % of general population (Coughlin et al. 1990). Abnormalities of appendix in CF include enlargement of outer diameter (>6 mm) in > 80 % of patients due to accumulation of inspissated mucoid content. It is important noting that these patients are asymptomatic, that no wall thickening or changes in appendiceal wall echopattern occur, and that involvement of periappendiceal and omental fat, and pain under gentle compression are absent (Lardenoye et al. 2004; Menten et al. 2005) (Fig. 2).
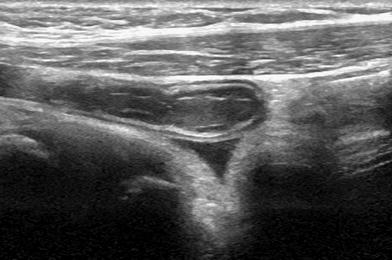

Fig. 2
Ultrasonographic features of appendiceal involvement in CF. The diameter is enlarged due to mucoid content and the typical inflammatory findings such as wall thickening, changes of the wall echopattern, hypertrophy of periappendiceal fat and pain under compression are absent
Another complication of CF is fibrosing colonopathy, a condition characterised by fibrosis of submucosa, predominantly involving the proximal portion of the colon, leading to the formation of local or pancolonic stenosis. Sonographic appearance of this abnormality is a diffuse thickened colon wall (up to 6.5 mm) with the maintenance of echo-stratification (Haber et al. 1997). The aetiology of this condition is uncertain. It does not seem to be correlated with intestinal inflammation (Pohl et al. 1997), previous meconium ileus, intestinal resection, distal intestinal obstruction syndrome, abdominal pain, or with high-dose pancreatic enzyme treatment (Haber et al. 1997), but it has been rather interpreted as an expression of glandular dysfunction in the CF intestine (Dialer et al. 2003). Another aspect of colonic wall in CF is colonic wall redundancy, also called jejunalisation of the colon. This condition, described for the first time in 2008, is more frequently seen in adult CF patients (it is described in up to 39 % of adult CF patients) and is usually asymptomatic or associated with non specific gastrointestinal symptoms (Webb et al. 2008; Caruso et al. 2012). Colonic wall redundancy is characterised at US by a hypertrophic colonic wall that appears wrinkled, doubled or tripled. Sometimes it is associated with pericolonic edema and mimics an intussusception.
2 Amyloidosis
Amyloidosis is a rare disease, characterised by forming pathological protein deposits—amyloid—in many organs and tissues. The deposition of amyloid is usually systemic, only 20 % of patients shows a localised, organ-specific, disease. Up to 15 % of the patients with myeloma are forming the deposits of amyloid. The primary amyloidosis affects both parenchymatous organs and the digestive tube. Mean age of the patients varies around 55–60 years. Renal insufficiency is a common sign. Heart, tongue, skin, kidney, nerves and the digestive tract are usually affected.
Amyloidosis may be inherited or acquired and can be also classified as primary or secondary. The primary amyloidoses have a familiar predisposition very often accompanied by the monoclonal gammopathy. The secondary or reactive amyloidoses occur as a complication of some long-lasting chronic inflammatory or tissue destructive diseases. The amyloid tends to form deposits in parenchymatous organs of the abdominal cavity and in lymph nodes.
Amyloidosis of the gastrointestinal tract is rare, occurring in 3.2 % of patients with all types of amyloidosis. It may be a dominant gastrointestinal involvement of a systemic amyloidosis (80 % of patients) or localised to the gastrointestinal tract without evidence of an associated plasma cell dyscrasia or other organ involvement (approximately 20 % of patients) (Cowan et al. 2012). Clinical symptoms depend on the actual location of the deposits. Weight loss, gastrointestinal bleeding, diarrhoea as well as intestinal obstruction, malabsorption, infarction and perforation may occur.
2.1 Ultrasonographic Features
The involvement of the small bowel in systemic forms of amyloidosis may be diffuse or very rarely focal. Rare cases of focal amyloidosis of the duodenum, jejunum and sigmoid colon have been described (Hirata et al. 1997; Matsui et al. 1996; Mainenti et al. 2010). These forms are characterised by amyloid infiltration of the entire intestinal wall thickness.
The sonographic features of intestinal amyloidosis may be non-specific, characterised by thickening of the bowel wall, mainly of the mucosa with hypoechoic and nodular plicae, decreasing of peristalsis, intestinal dilatation and mesenteric lymph nodes with diameter ranging between 10 and 20 mm (Mainenti et al. 2010). Thickened bowel walls did not show significant increase in vascularisation. In particular, the thickened intestinal walls are more frequently remarkably hypovascularised, and dilated vascular structures can be visible within the mesentery (Fig. 3).
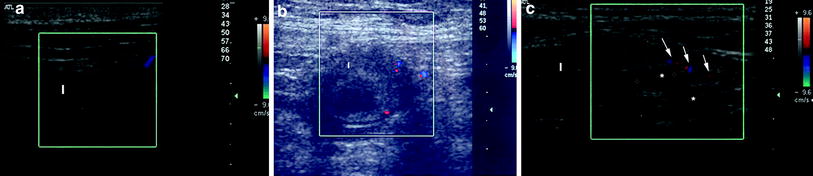

Fig. 3
Ultrasonographic features of small bowel amyloidosis, characterised by wall thickening (arrows) associated with decreased vascularity at color Doppler (a, b) and (c) enlarged mesenteric lymph nodes (asterisks) and mesenteric hypertrophy with dilated vascular structures (arrows). I, Ileum
Focal dilatation of the small bowel or gastric lumen associated to wall thickening may represent a radiological sign for suggesting intestinal amyloidosis in the differential diagnosis (Fig. 4; Kala et al. 2007). In fact, amyloid may form deposits within the neural myenteric plexus, which determine the hypotonia of the gastrointestinal wall and promote the pseudoaneurysmal dilatation (Araoz et al. 2000).
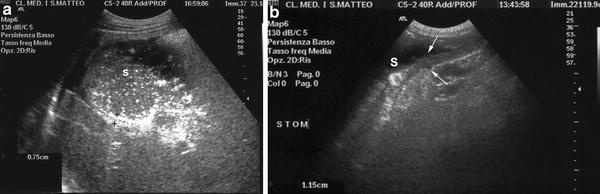

Fig. 4
a Dilatation of the stomach (S) associated to wall thickening (arrows) with absence of peristalsis b in a patient with gastric amyloidosis
3 Vasculitis
Vasculitis includes a group of inflammatory disorders that affect both arteries (arteritis) and veins (phlebitis). It is primarily due to the leukocyte migration and to the resultant damage of blood vessels, and can be classified according to the cause, location, type and the size of the vessels (Gardner-Medwin et al. 2002).
Vasculitis may present with systemic symptoms such as fever and weight loss, skin alterations such as palpable purpura and livedo reticularis and gastrointestinal manifestations such as abdominal pain, diarrhea, bloody stool and perforation.
Vasculitides that most commonly affect the gastrointestinal system are Bechet’s disease, Henoch Shoenlein purpura and polyarteritis nodosa.
Bechet’s disease usually presents with abdominal pain, flatulence, diarrhea, constipation, mucus or blood in the stool, and intestinal ulcers.
Henoch Shoenlein purpura is characterised by abdominal pain, nausea, vomiting, diarrhea or constipation and in one third of patients by gastrointestinal bleeding and intestinal intussusception.
Polyarteritis nodosa shows abdominal pain due to the damage to the medium- and small-sized arteries of the bowel.
3.1 Ultrasonographic Features
Various ultrasonographic features have been described in the vasculitides, depending on the specific disease. However, vasculitides share common ultrasonographic features such as bowel wall thickening, loss of bowel stratification and focal dilatation of the bowel, due to their vascular and inflammatory pathogenesis.
These features are present in Henoch-Shoenlein purpura, where sometime can be also observed an extra-echoic layer internal to the submucosa that may be due to the bleeding within the deep mucosa and submucosa. These changes may be also the result of bowel wall oedema due to vasculitis of the supplying vessels (Fig. 5; Couture et al. 1992; Nchimi et al. 2008).