With the rapid and widespread adoption of minimally invasive procedures (laparoscopic and robotic) for the treatment of prostate and bladder cancers in the last decade, concerns have been raised regarding whether the technique can emulate the time-tested gold standard open procedures. This article briefly reviews the indications for lymph node dissection for bladder and prostate cancer, and reviews the role of extended lymphadenectomy in each procedure. Much of the focus of this review is on minimally invasive approaches and the technical aspects of the procedures, the feasibility of the robotic technique, and early oncologic outcomes.
This article is not certified for AMA PRA Category 1 Credit ™ because product brand names are included in the educational content. The Accreditation Council for Continuing Medical Education requires the use of generic names and or drug/product classes as the required nomenclature for therapeutic options in continuing medical education.
For more information, please go to www.accme.org and review the Standards of Commercial Support.
With the rapid and widespread adoption of minimally invasive procedure (specifically robotic assistance in the United States) for these two cancers, concerns have been raised regarding whether the technique can emulate the time-tested gold standard open procedures. These concerns are not only directed to the surgical margins and resection of the primary tumor, but also question whether an adequate node dissection along the same defined “extended” templates and hence nodal counts can be achieved robotically. Rightfully so, concerns have been raised by the urologic oncology community regarding whether lymph node dissection is even performed in every case for which it is indicated, especially in prostate cancer.
In this article the authors briefly review the indications for lymph node dissection for bladder and prostate cancer, and review the role of extended lymphadenectomy (LND) in each procedure. Other articles elsewhere in this issue are dedicated to the role of LND for both prostate and bladder cancer. Therefore, much of the focus of this review is on minimally invasive approaches and the technical aspects of the procedures, the feasibility of the robotic technique, and early oncologic outcomes.
Role of lymphadenectomy in prostate cancer
The role of LND in prostate cancer remains a controversial one, both in terms of the indications as well as the extent. Most investigators agree that LND offers significant pathologic and prognostic information, which affects the use of adjuvant therapy. Prostate-specific antigen (PSA) screening has resulted in a downward stage migration in prostate cancer within the past several years. By detecting disease at an earlier stage, the likelihood of lymph node metastases has decreased and most contemporary series place the lymph node detection rate at 5% for the conventional standard lymph node dissection (obturator/external iliac). Many surgeons currently omit LND in patients with low-grade (Gleason score ≤6), low-stage (cT2a or less) prostate cancer, or when the likelihood of lymph node metastases is less than 2% based on the Partin tables or Kattan nomograms.
Overall decline in lymphadenectomy for prostate cancer
This stage migration likely explains the overall decline in the performance of LND in the United States over the past few decades. For example, the Prostate Strategic Urologic Research Endeavor database previously demonstrated a trend toward omitting pelvic LND (PLND) for low-risk and intermediate-risk prostate cancer from 94% in 1992 to 80% in 2004, with a mean lymph node yield of 5.7 (median 5). The use of LND in the minimally invasive prostate literature has declined even more rapidly, as is evidenced by two recent population-based studies. Prasad and colleagues reviewed the Surveillance, Epidemiology, and End Results (SEER) from 2003 to 2005 to assess factors correlating with the performance of LND. The investigators looked at both laparoscopic radical prostatectomy (LRP) and robotic-assisted laparoscopic prostatectomy (RALP), although the time frame correlated with the early adoption of RALP. A multivariate analysis assessing surgical approach, volume, patient demographics, comorbidity, and geographic region was conducted. Overall, 68% of men underwent PLND, with the rates varying significantly by surgical approach (17% vs 83% for minimally invasive vs open prostatectomy, respectively, P <.001). High-volume surgeons were more likely to perform PLND in both groups. Of note, minimally invasive surgeons performed PLND in only 28% of cases. The investigators offered several reasons for this discrepancy in performing PLND during the early RALP era, including the new approach and the desire to shorten the operating times, reduce complications, and lessen costs. Another overriding factor suggested was that most patients undergoing surgery in the present era have low-risk cancer predicted to have a low rate of nodal metastases, persuading many surgeons to adopt the attitude that PLND is simply unnecessary.
Feifer and colleagues recently reviewed the SEER database for PLND trends for both open and minimally invasive prostatectomy from 2003 to 2007. The investigators found that the use of PLND declined over time both overall and within subgroups (including those with high-risk features). PLND was 5 times more likely in men undergoing radical retropubic prostatectomy (RRP) compared with the minimally invasive cohort (both laparoscopic and robotic approaches). While elevated PSA and Gleason sum were significant predictors of LND in both groups, the magnitude was significantly greater for RRP. Only 60% of patients in the minimally invasive cohort with PSA greater than 10 underwent LND, compared with 86% of those in the open group. The investigators were not able to assess the number of lymph nodes removed or the anatomic extent of LND.
Overall decline in lymphadenectomy for prostate cancer
This stage migration likely explains the overall decline in the performance of LND in the United States over the past few decades. For example, the Prostate Strategic Urologic Research Endeavor database previously demonstrated a trend toward omitting pelvic LND (PLND) for low-risk and intermediate-risk prostate cancer from 94% in 1992 to 80% in 2004, with a mean lymph node yield of 5.7 (median 5). The use of LND in the minimally invasive prostate literature has declined even more rapidly, as is evidenced by two recent population-based studies. Prasad and colleagues reviewed the Surveillance, Epidemiology, and End Results (SEER) from 2003 to 2005 to assess factors correlating with the performance of LND. The investigators looked at both laparoscopic radical prostatectomy (LRP) and robotic-assisted laparoscopic prostatectomy (RALP), although the time frame correlated with the early adoption of RALP. A multivariate analysis assessing surgical approach, volume, patient demographics, comorbidity, and geographic region was conducted. Overall, 68% of men underwent PLND, with the rates varying significantly by surgical approach (17% vs 83% for minimally invasive vs open prostatectomy, respectively, P <.001). High-volume surgeons were more likely to perform PLND in both groups. Of note, minimally invasive surgeons performed PLND in only 28% of cases. The investigators offered several reasons for this discrepancy in performing PLND during the early RALP era, including the new approach and the desire to shorten the operating times, reduce complications, and lessen costs. Another overriding factor suggested was that most patients undergoing surgery in the present era have low-risk cancer predicted to have a low rate of nodal metastases, persuading many surgeons to adopt the attitude that PLND is simply unnecessary.
Feifer and colleagues recently reviewed the SEER database for PLND trends for both open and minimally invasive prostatectomy from 2003 to 2007. The investigators found that the use of PLND declined over time both overall and within subgroups (including those with high-risk features). PLND was 5 times more likely in men undergoing radical retropubic prostatectomy (RRP) compared with the minimally invasive cohort (both laparoscopic and robotic approaches). While elevated PSA and Gleason sum were significant predictors of LND in both groups, the magnitude was significantly greater for RRP. Only 60% of patients in the minimally invasive cohort with PSA greater than 10 underwent LND, compared with 86% of those in the open group. The investigators were not able to assess the number of lymph nodes removed or the anatomic extent of LND.
Extended lymphadenectomy for high-risk disease
Despite this trend against LND for men with lower risk of disease, there has been a movement in performing more extended LNDs for those patients deemed worthy based on risk stratification, with significantly higher lymph node/positive lymph node yields. The rationale is that higher-risk patients are more likely to harbor disease that has spread to regional lymph nodes. Further, many of these nomograms and tables are based on a PLND, consisting of the obturator fossa and external iliac regions. Briganti and colleagues have published nomograms predicting the probability of lymph node metastasis among patients undergoing extended PLND (ePLND), which await external validation.
Schumacher and colleagues analyzed their technique regarding long-term outcomes of 122 patients undergoing ePLND (≥10 nodes removed) followed by RRP for high-risk disease (median PSA 16 ng/mL). The lymphatic tissue is taken from 3 distinct locations: the external iliac vein, obturator fossa, and internal iliac artery. Seventy-six percent had Stage pT3 disease or greater and 50% had seminal vesicle invasion. Positive lymph nodes were located exclusively along the external iliac vein in 9% of patients, in the obturator fossa in 16.4%, and along the internal iliac artery (presacral lymph nodes) in 21.3%. In approximately half of the patients, positive lymph nodes were found along the internal iliac vessels, along with positive lymph nodes in the area of the obturator fossa and/or the external iliac vein. Overall, positive internal iliac lymph nodes alone or combined with positive lymph nodes in other locations were found in 70.5% of patients. The median cancer-specific survival rate at 5 and 10 years was 84.5% and 60.1%, respectively. For patients with 2 or less or 3 or more positive lymph nodes removed, the median cancer-specific survival rate at 10 years was 78.6% and 33.4%, respectively ( P <.001).
Lymphadenectomy in laparoscopic prostatectomy
Despite a lower incidence of PLND in the minimally invasive literature, there are several studies assessing the outcomes for both standard PLND (sPLND) and ePLND. LRP was introduced in the late 1990s, with several larger series and reviews showing comparable oncologic outcomes to the RRP. Most LRP series report sPLNDs performed in similar fashion to the RRP, with comparable results and complications.
Eden and colleagues evaluated 374 patients who underwent LRP with an LND. Two hundred and fifty-three men had an sPLND and 121 had an ePLND for intermediate-risk or high-risk prostate cancer. An extraperitoneal approach was used in all patients having sPLND and a transperitoneal approach in patients having ePLND. The investigators found that the ePLND group had a greater percentage of patients with cT3 disease (9.9% vs 4.2%, P = .046), and was associated with a longer operating time of 206.5 versus 180.0 minutes ( P <.001) and a higher node count of 17.5 versus 6.1 ( P = .002). Blood loss, hospital stay, transfusion, and complication rates were similar in the two groups.
Wyler and colleagues reported on their experience with ePLND in 123 patients undergoing LRP for high-risk prostate cancer. The boundaries of the pelvic lymph node dissection were the bifurcation of the common iliac artery superiorly, the node of Cloquet inferiorly, the external iliac vein laterally, and the bladder wall medially. The mean number of lymph nodes removed was 21 (range 9–55) in a mean PLND time of 47 minutes. A total of 21 patients (17%) had lymph node metastases. The overall complication rate was 4%. Associated complications occurred in 1 patient (0.8%) with an iliac hematoma and postoperative paresis of the leg that resolved spontaneously, and 1 patient (0.8%) with a lymphocele (after extraperitoneal laparoscopic operation with no primary drainage) that was drained. Two patients (1.6%) had lymphedema of the right leg that resolved spontaneously within 2 and 4 months. Similarly, Lattouf and colleagues showed that ePLND was feasible in 35 patients undergoing LRP for high-risk prostate cancer (median PSA 16.5). The template included the genitofemoral nerve up to the bifurcation of the common iliac artery and down to the epigastric artery. The investigators retrieved a median of 13 lymph nodes. In 5 of the 11 patients with positive lymph nodes, the nodes were detected exclusively outside the obturator fossa. The complications were 2 temporary and reversible neurapraxias (ischiatic nerve and obturator nerve), 1 deep vein thrombosis, and 2 lymphoceles. One lymphocele healed conservatively; the second was marsupialized laparoscopically.
In the United States there has been a significant increase in the number of RALPs performed in the past decade. As surgeons have become more familiar with RALP, a larger volume of patients with greater PSA values, Gleason scores, and clinical stages are being offered robotic surgery. In these patients with more aggressive disease characteristics, concomitant PLND is traditionally performed for the aforementioned reasons.
Lymphadenectomy in robotic prostatectomy
The feasibility of LND within RALP is well established, with several large series demonstrating comparable yield and perioperative outcomes to the RRP. Zorn and colleagues compared 296 RALP patients undergoing standard PLND (external iliac and obturator fossa) with 859 patients undergoing RALP alone. The investigators removed a mean number of 12.5 lymph nodes. The mean operative time (224 vs 216 minutes; P = .09), estimated blood loss (206 vs 229 mL; P = .14), and hospital stay (1.32 vs 1.24 days; P = .46) were comparable between the two groups. The rate of intraoperative complications (1% vs 1.5%; P = .2), overall postoperative complications (9% vs 7%; P = .8), and lymphocele formation (2% vs 0%; P = .9) were not significantly different. The rates compared well with the historical RRP experience. These investigators and many others hypothesize that the low rate of lymphoceles in the RALP literature may be due to increasing technical experience as well as the transperitoneal nature of RALP, which allows for easier egress of the lymphatic fluid, thus preventing collection formation. It has to be noted, however, that because routine imaging is not performed after prostatectomy, the incidence of subclinical lymphoceles might be higher than expected. Even in transperitoneal procedures, there is a “resealing” of the peritoneum expected after 48 to 72 hours in an uncomplicated procedure, which can still potentially mediate lymphocele formation by preventing transperitoneal absorption of lymphatic fluid.
In a feasibility study using graded complications, Yee and colleagues assessed the complications and nodal yield of ePLND for 32 patients undergoing RALP for localized disease (all risk categories). The investigators included the obturator, hypogastric, external iliac, and common iliac lymph nodes up to the bifurcation of the aorta in their dissection. The median number of lymph nodes retrieved was 18, with 4 patients having lymph node metastases. Median operative time for the ePLND was 72 minutes (interquartile range 66–86). Graded complications included 13 grade 1 events and 1 grade 2 event, with 1 grade 1 event being considered related to ePLND. No clinically presenting lymphoceles or thrombotic events were encountered.
Feicke and colleagues also reported the feasibility of ePLND in 99 patients undergoing RALP for PSA of 10 or more or Gleason score greater than 7. The surgeons operated for an average of 51 minutes with a median number of 19 lymph nodes removed. Lymphedema was observed in 2 patients: in one patient, a bilateral lymphedema of the lower leg resolved after physical therapy with supportive lymphatic drainage; the other patient showed persistent unilateral lymphedema at a 3-month follow-up visit. In 2 patients, a symptomatic lymphocele was treated conservatively, and 2 patients required percutaneous drainage ( Table 1 ).
| Authors, Ref. Year | Approach | No. of Patients | Median No. of LNs Removed (Range) | Proximal Extent of Dissection | Minutes for ePLND (Range) | Lymphadenectomy-Specific Complications |
|---|---|---|---|---|---|---|
| Feicke et al, 2009 | RALP | 99 | 19 (8–53) | Iliac bifurcation | 51 (29–81) | Lymphocele (4) Lymphedema (2) |
| Yee et al, 2010 | RALP | 32 | 18 (IQR 12–28) | Aortic bifurcation | 72 (IQR 66–86) | Grade 1 (1) |
| Eden et al, 2010 | LRP | 121 | 17.5 (2–23) | Iliac bifurcation | Additional 26 min (compared with sPLND) | Obturator nerve injury (2) |
| Wyler et al, 2006 | LRP | 123 | 21 (9–55) | Iliac bifurcation | 47 min | Iliac hematoma (1) Lymphocele requiring intervention (1) Temporary lymphedema (2) |
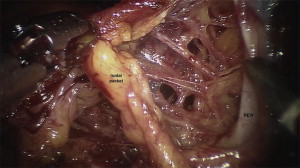
The extent of the lymph node dissection for prostate cancer with robotic prostatectomy can easily emulate the open operation (discussed in an article by La Rochelle and Amling elsewhere in this issue). The robotic camera allows for excellent visualization and identification of individual lymphatic channels for ligation or bipolar cauterization ( Fig. 1 ). The cephalad extent of the dissection is often the iliac bifurcation. The distal extent is the node of Cloquet. The posterior extent is below the obturator vein. Laterally the dissection extends to the external iliac artery. In this fashion the obturator nodes, internal and external iliac nodes, and the common iliac nodes up to the iliac bifurcation can be removed ( Fig. 2 ).