Pelvic lymph node dissection is the only reliable technique to detect low-volume lymph node involvement in prostate cancer. Extended lymph node dissections that include the internal iliac chain in addition to the external iliac and obturator packets have shown a significantly higher proportion of patients to have lymphatic involvement than previously recognized. The improved staging afforded by a more extended dissection raises several questions. Addressing these questions is the focus of this review.
As a staging modality, pelvic lymph node dissection remains an important component of radical prostatectomy (RP) due, in part, to the low sensitivity of preoperative imaging studies. CT and MRI are able to detect approximately only 40% of cases with low-volume nodal involvement. Ferumoxtran, an MRI-based intravenous lymph node imaging agent, has been used to demonstrate microscopic nodal deposits with impressive results, but it remains unapproved by the US Food and Drug Administration. Additionally, examining the images of each lymph node for the small filling defects that indicate a metastatic deposit is time consuming. Fluorocholine-based positron emission tomography has shown some potential but it is still an experimental technique with questionable sensitivity. Thus, for the foreseeable future, pelvic node dissection remains the most reliable technique for the detection of low-volume nodal involvement in prostate cancer.
The last article in Urologic Clinics of North America to discuss the indications of pelvic lymph node dissection for prostate cancer was in 2001. The focus of that review and most relevant urologic literature at that time was on the effort to identify patients at very low risk for lymph node metastasis who could be spared a node dissection with its additional operative time and morbidity. Given that a node dissection was considered primarily a staging method rather than a therapeutic one, it was generally believed that the staging accuracy afforded by the preoperative clinical variables of prostate-specific antigen (PSA), biopsy Gleason score, and clinical tumor stage (ie, Partin tables) was often adequate for low-risk patients. Accumulating data since that review has challenged previous beliefs. Extended lymph node dissections that include the internal iliac chain in addition to the external iliac and obturator packets ( Fig. 1 ) have shown a significantly higher proportion of patients to have lymphatic involvement than previously recognized.
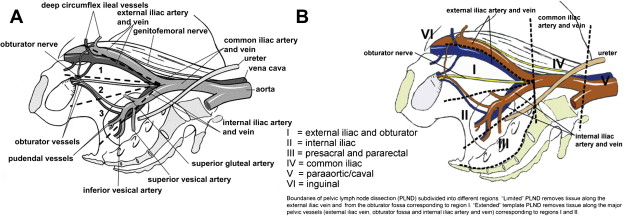
There is now little doubt that an extended node dissection detects more patients with lymph node involvement (LNI) compared with a limited dissection. The improved staging afforded by a more extended dissection raises several questions. First, are there still patients at very low risk for LNI who can be spared a lymph node dissection? Second, is there a therapeutic benefit to an extended dissection? Third, what are the drawbacks of doing an extended lymph node dissection? Fourth, should patients with nodal involvement on an extended dissection be treated the same as patients with nodal disease on a limited dissection (ie, Should they be excluded from adjuvant radiation and/or be offered immediate androgen-deprivation therapy)? Lastly, what are the current recommendations from the American Urological Association (AUA), the European Association of Urology (EAU), and the National Comprehensive Cancer Network (NCCN) regarding patient selection and extent of dissection? Addressing these questions is the focus of this review, although some cannot be definitively answered given the current limitations of the literature.
Evidence for improved staging by extended lymph node dissection
There has been a growing appreciation over the past 10 years that a limited pelvic lymph node dissection (limPLND) at the time of RP, which removes nodes from only the external iliac and obturator locations, can miss a significant percentage of potentially cancer-bearing nodal tissue. A mapping study by Mattei and colleagues using a radiolabeled tracer injected into the prostate followed by single-photon emission CT scanning, intraoperative lymphatic mapping with a gamma probe, and a superextended lymph node dissection extending above the aortic bifurcation demonstrated that the primary lymphatic landing sites of the prostate can also include presacral, pararectal, paracaval, para-aortic, and even inguinal lymph nodes ( Fig. 2 ). The investigators calculated that a limPLND includes only 38% of prostate-draining nodes and that an extended pelvic lymph node dissection (ePLND), including the tissue along the internal and distal common iliac vessels, would remove 75%. This study did not report on the distribution of patients with exclusive drainage to basins outside the limited and extended dissection templates who might therefore be understaged by the different pelvic lymph node dissection (PLND) schema. A report by Schumacher and colleagues of 122 node-positive patients described the likelihood of having positive lymph nodes outside the limPLND template, exclusively or in combination with other sites. Overall, 70% had positive nodes in the internal iliac basin, and 21% were positive only in this area. This compared with 9% positive only in the external iliac distribution and 16% positive only in the obturator fossa. It is not surprising that Heidenreich and colleagues reported a significantly higher rate of positive lymph nodes in 103 patients undergoing RP with ePLND compared with 100 patients undergoing RP with limPLND (26% vs 12%) who were otherwise well matched for Gleason score, clinical tumor stage, and PSA. Likewise, Touijer and colleagues reported a hazard ratio of 7.15 for positive nodes in patients undergoing an ePLND compared with those undergoing only an external iliac dissection in a group with a greater than 1% risk for nodal involvement based on the Partin tables.
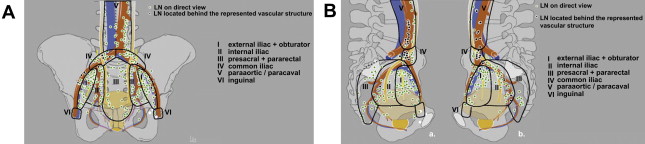
Is there still a low-risk population that can be spared a lymph node dissection?
It is well established that an ePLND demonstrates a higher rate of LNI compared with a limPLND. Does this finding extend, however, to patients previously considered low-risk for LNI based on preoperative variables? In a large series of men from Johns Hopkins treated with RP over the past 30 years, the rate of positive lymph nodes decreased from 11% in 1982 through 1991 to 1% in 2000 through 2005. The decline was due in part to migration toward lower stages because of increasing use of PSA screening, but there also were decreasing rates of positive lymph nodes within the predefined risk categories based on PSA, Gleason score, and clinical tumor stage. This led to questions about the utility of a lymph node dissection in the large segment of men undergoing surgery for low-risk disease. Cagiannos and colleagues developed a nomogram using standard preoperative variables with the goal of identifying men at very low risk of nodal involvement who could be spared a PLND, and community-based data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) demonstrated decreasing rates of PLND at the time of RP.
With the awareness that removing more nodes can lead to increased detection of nodal metastases, is there still a substantial segment of men who are still at very low risk for LNI at ePLND, or is the prevalence of occult positive nodes in men with prostate cancer high enough that nearly all patients should undergo an ePLND? This question cannot be answered in an absolute sense because the underlying characteristics of a patient population determine how often LNI would be found on ePLND. Additionally, patients’ and practitioners’ tolerance for the risk of understaging is potentially variable. Using a reasonable population estimate of the incidence of LNI at RP with ePLND, however, along with an acceptable arbitrary cutoff for the risk of LNI in an individual patient that should trigger an ePLND, it can begin to be determined which and how many patients could safely have an ePLND omitted with low risk of understaging. Godoy and colleagues from Memorial Sloan-Kettering Cancer Center did just this using a recalibrated nomogram based on findings from 3721 patients undergoing RP with ePLND. Given an overall 7% incidence of LNI, along with an individual cutoff of greater than or equal to 2% (ie, patients with nomogram-calculated risk of positive nodes greater than or equal to 2% would undergo ePLND), the investigators estimated that approximately 35% of patients could be spared an ePLND by using their nomogram. In their study population, which had an actual 5.2% incidence of LNI, 53% of patients were categorized as low risk and less than 1% of that group had positive nodes at ePLND. Briganti and colleagues developed a similar nomogram based on 602 patients undergoing RP with ePLND. One aspect that potentially makes it difficult to use this nomogram is that their absolute lowest measurable probability of LNI was 2%, which is likely due to the higher rate of LNI (11%) in their study. This higher LNI rate is attributable either to a difference in populations or to the fact that the mean number of nodes removed in their report was 17 compared with 11 in the study by Godoy and colleagues.
There has been concern that using preoperative variables may underestimate the true risk of LNI at the time of ePLND. Studies that demonstrated higher than expected LNI in patients with PSA less than or equal to 10 or a biopsy Gleason score less than or equal to 6 used methods that are difficult to compare with current practice. Biopsy templates were not reported, and fine-needle aspiration was used in one study. In the report by Weckermann and colleagues of higher than expected LNI in patients with Gleason score less than or equal to 6 and PSA less than or equal to 10 (5.4%–11.3%), there was a substantial rate of upgrading and/or upstaging at surgery (46%–80%). In a report by Burkhard and colleagues, 23% of patients with pathologic Gleason score of 5 and 25% with Gleason score of 6 prostate cancer had LNI. The median PSAs of these groups, however, were 11 and 13, respectively. An updated report from this group found that among patients with both pathologic Gleason score of 5 or 6 and PSA less than or equal to 10, the rate of LNI was 4.5%. No clinical tumor stage was given for these patients. Therefore, it seems that in ostensibly low-risk patients with LNI, there are often other clinical risk factors present that would remove many, but not all, from the low-risk category.
In conclusion, it seems that in spite of a higher rate of LNI at RP than was previously recognized, there is still a significant number of men who would not be understaged by omission of a PLND. That number is smaller than previously appreciated, however. For men with a higher risk of LNI, an ePLND is a significantly better method of staging, although the cutoff risk level that should trigger an ePLND is somewhat arbitrary. Nomograms or risk categories that predict the probability of LNI found with limPLND underestimate the actual risk of LNI, and ePLND-based prediction tools are preferable.
Is there still a low-risk population that can be spared a lymph node dissection?
It is well established that an ePLND demonstrates a higher rate of LNI compared with a limPLND. Does this finding extend, however, to patients previously considered low-risk for LNI based on preoperative variables? In a large series of men from Johns Hopkins treated with RP over the past 30 years, the rate of positive lymph nodes decreased from 11% in 1982 through 1991 to 1% in 2000 through 2005. The decline was due in part to migration toward lower stages because of increasing use of PSA screening, but there also were decreasing rates of positive lymph nodes within the predefined risk categories based on PSA, Gleason score, and clinical tumor stage. This led to questions about the utility of a lymph node dissection in the large segment of men undergoing surgery for low-risk disease. Cagiannos and colleagues developed a nomogram using standard preoperative variables with the goal of identifying men at very low risk of nodal involvement who could be spared a PLND, and community-based data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) demonstrated decreasing rates of PLND at the time of RP.
With the awareness that removing more nodes can lead to increased detection of nodal metastases, is there still a substantial segment of men who are still at very low risk for LNI at ePLND, or is the prevalence of occult positive nodes in men with prostate cancer high enough that nearly all patients should undergo an ePLND? This question cannot be answered in an absolute sense because the underlying characteristics of a patient population determine how often LNI would be found on ePLND. Additionally, patients’ and practitioners’ tolerance for the risk of understaging is potentially variable. Using a reasonable population estimate of the incidence of LNI at RP with ePLND, however, along with an acceptable arbitrary cutoff for the risk of LNI in an individual patient that should trigger an ePLND, it can begin to be determined which and how many patients could safely have an ePLND omitted with low risk of understaging. Godoy and colleagues from Memorial Sloan-Kettering Cancer Center did just this using a recalibrated nomogram based on findings from 3721 patients undergoing RP with ePLND. Given an overall 7% incidence of LNI, along with an individual cutoff of greater than or equal to 2% (ie, patients with nomogram-calculated risk of positive nodes greater than or equal to 2% would undergo ePLND), the investigators estimated that approximately 35% of patients could be spared an ePLND by using their nomogram. In their study population, which had an actual 5.2% incidence of LNI, 53% of patients were categorized as low risk and less than 1% of that group had positive nodes at ePLND. Briganti and colleagues developed a similar nomogram based on 602 patients undergoing RP with ePLND. One aspect that potentially makes it difficult to use this nomogram is that their absolute lowest measurable probability of LNI was 2%, which is likely due to the higher rate of LNI (11%) in their study. This higher LNI rate is attributable either to a difference in populations or to the fact that the mean number of nodes removed in their report was 17 compared with 11 in the study by Godoy and colleagues.
There has been concern that using preoperative variables may underestimate the true risk of LNI at the time of ePLND. Studies that demonstrated higher than expected LNI in patients with PSA less than or equal to 10 or a biopsy Gleason score less than or equal to 6 used methods that are difficult to compare with current practice. Biopsy templates were not reported, and fine-needle aspiration was used in one study. In the report by Weckermann and colleagues of higher than expected LNI in patients with Gleason score less than or equal to 6 and PSA less than or equal to 10 (5.4%–11.3%), there was a substantial rate of upgrading and/or upstaging at surgery (46%–80%). In a report by Burkhard and colleagues, 23% of patients with pathologic Gleason score of 5 and 25% with Gleason score of 6 prostate cancer had LNI. The median PSAs of these groups, however, were 11 and 13, respectively. An updated report from this group found that among patients with both pathologic Gleason score of 5 or 6 and PSA less than or equal to 10, the rate of LNI was 4.5%. No clinical tumor stage was given for these patients. Therefore, it seems that in ostensibly low-risk patients with LNI, there are often other clinical risk factors present that would remove many, but not all, from the low-risk category.
In conclusion, it seems that in spite of a higher rate of LNI at RP than was previously recognized, there is still a significant number of men who would not be understaged by omission of a PLND. That number is smaller than previously appreciated, however. For men with a higher risk of LNI, an ePLND is a significantly better method of staging, although the cutoff risk level that should trigger an ePLND is somewhat arbitrary. Nomograms or risk categories that predict the probability of LNI found with limPLND underestimate the actual risk of LNI, and ePLND-based prediction tools are preferable.
Is an extended lymph node dissection potentially therapeutic?
The question looming over much of the discussion surrounding ePLND is whether finding and removing limited metastatic deposits in pelvic lymph nodes can potentially cure patients or at least measurably delay progression. Prostate cancer does not follow a routine progression to the regional lymph nodes before systemic dissemination, as is evidenced by the surprising rate of PSA-positive epithelial cells in bone marrow aspirates of patients with otherwise pathologically organ-confined disease. Patients with a negative PLND have a better prognosis than those with LNI, but node-negative status is not associated with durable recurrence-free survival to the same extent as in urothelial, germ cell, or penile tumors. There are no prospective, randomized trials that have compared the outcome of patients with or without PLND. Nevertheless, there is some indirect evidence that patients with limited LNI or with negative nodes may get a therapeutic benefit from a node dissection. These data, however, must be interpreted with caution. A report from Josyln and Konety describing results from the Surveillance, Epidemiology and End Results (SEER) database is often cited by proponents of the therapeutic benefit of PLND. In that report, the authors demonstrated a survival advantage for both node-negative and node-positive patients when greater than 4 nodes were removed compared with those having fewer nodes removed. A key point that should not be overlooked is that this survival benefit can potentially be attributed to the so-called Will Rogers effect, which can occur when comparing outcomes in patients who have been staged differently. Patients remaining in the node-negative group after having a more extensive node dissection are more likely to be truly node negative and therefore have a better outcome compared with those included in the node-negative category after a limited dissection, some of whom are likely harboring occult LNI. The same holds true for the node-positive group, whereby those included in the node-positive group after an extensive node dissection may have less overall disease and therefore a better prognosis than patients found to have nodal metastases after a limited dissection who likely have more extensive disease. The paradoxic effect of this phenomenon is that by transferring patients with limited LNI out of the node-negative group and into the node-positive group through better identification (more extensive PLND), both node-negative and node-positive patients seem to do better than historical controls, in spite of there having been no change in overall or individual outcomes. In that same report, node-negative patients with more than 10 nodes removed also had a cancer-specific survival (CSS) advantage, which was attributed to the possibility that micrometastases were removed. This could also be explained, however, by the Will Rogers effect. Likewise, a retrospective study by Masterson and colleagues that found improved recurrence-free survival in node-negative patients with higher number of removed nodes could similarly be explained.
Several investigators have retrospectively reported on large single-institution experiences with the aim of elucidating a possible therapeutic benefit for PLND. Weight and colleagues updated the Cleveland Clinic experience with Kaplan-Meier 10-year estimated biochemical relapse (BCR)-free survival in favorable risk patients with (n = 140) and without (n = 196) a limPLND. No difference was detected (88% vs 84%, P = .33). The investigators admitted they could not specifically comment on the value of an ePLND and that their study may have been underpowered to detect a small benefit to PLND. DiMarco and colleagues described the Mayo Clinic experience with 7036 node-negative patients undergoing RP with PLND between 1987 and 1999. The average number of lymph nodes removed steadily declined during that time period, from 14 in 1987 to 5 in 1999. A multivariate analysis of outcome based on grade, clinical stage, PSA, year of surgery, and number of nodes removed failed to show any BCR-free survival benefit in any risk category for increased number of nodes removed at PLND. Presumably, removing fewer nodes would leave more residual occult positive nodes and lead to higher rates of progression, particularly in higher risk disease, but this was not seen. Lastly, Allaf and colleagues from Johns Hopkins reported on BCR-free survival in patients with LNI found at RP by two high-volume surgeons, one who routinely performed a limPLND and one who performed an ePLND. There was a trend toward improved BCR-free survival in patients with LNI undergoing ePLND (n = 71) versus limPLND (n = 22) (34.4% vs 16.5%, P = .07). A significant difference in BCR-free survival was reached in the subgroup with LNI but less than 15% of nodes involved (42.9% vs 10%, P = .01). The number of nodes removed by each surgeon was not significantly different in this subgroup (15.9 vs 15.1) in spite of the difference in extent of dissection. Even though this report is encouraging with respect to the possibility of a therapeutic effect, there is still a distinct chance that the Will Rogers effect is at work here. More patients in the ePLND group may truly have had limited LNI due to a wider sampling compared with the group with limited LNI based on a limPLND, many of whom may have had a higher actual burden of LNI.
Although there is no conclusive evidence that a PLND results in improved prostate cancer outcomes, a significant percentage of men with LNI across several studies consistently have prolonged BCR-free survival without adjuvant therapy, even men undergoing limPLND. Five-year to-10-year BCR-free rates range from 4% to 40% in these reports, with patients with fewer positive nodes consistently having a better prognosis. It is difficult to imagine an explanation for this outcome other than a low but significant percentage of men with low-volume LNI can be cured of their disease or at least have a long-term delay in progression. Extensive lymph node dissection in bladder cancer, a more aggressive pelvic cancer with an overlapping lymphatic drainage pattern, can also cure some patients with limited LNI. It should, therefore, not be surprising that the same seems true in prostate cancer, although admittedly an ePLND in prostate cancer is not as extensive as the typical dissection in bladder cancer.
Without a prospective randomized trial of ePLND versus limited or no PLND, it is impossible to unequivocally answer the question of whether an ePLND results in a measurable therapeutic benefit. Until such data exist, retrospective analyses, with all of their inherent selection bias, must be relied on. Most reports consistently show a reasonable percentage of men with LNI with long-term recurrence-free survival, however, which should at least prompt strong consideration of a PLND. Given that an ePLND is more likely to remove cancer-bearing nodes in patients at higher risk of LNI, it is likely that any possible therapeutic advantage to PLND will be greater in an ePLND.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








