The history of urologic lymphadenectomy is rich and diverse. Our current understanding of its use and benefits is a product of the hard work of numerous physicians and scientists from many nations. Standard dissection templates for the various urologic malignancies are based on a complete understanding of the anatomy of the lymphatic system, which has developed immensely since Hippocrates first described the white blood of the lymphatic system while performing an axillary dissection. It is hoped that the next 100 years will bring even greater comprehension of its value and utility.
History of the lymphatic system
The history of the lymphatic system dates back to the biblical era. There are many references in both the early Hebrew and Greek literature to severe swelling of the lower extremities. Unknowingly, these authors described afflictions of the lymphatic system that would only be comprehended millennia later. Hippocrates (460–370 bc ) was the first to actually describe the lymphatic system itself. After performing axillary lymph node dissections he commented on the “white blood” contained within them. Aristotle (384–322 bc ) went on to describe “fibers which course between blood vessels and nerves and contain clear liquid.” Celsus (30 bc to 50 ad ) was the first person to name a symptom complex associated with lymphatic dysfunction and is credited with the term elephantiasis, which he used to describe persons with extreme edema of the lower extremities because it resembled the hide of an elephant. The first to describe lymphatics associated with an organ system was Erasistratus (310–250 bc ), a respected anatomist of Alexandria, who described “white arteries” of the small bowel. Surprisingly, Vesalius, who is considered the founder of modern anatomy and who described the vascular system in detail, made no mention of the lymphatic system. It was not until 1622 that an anatomist had accurately dissected and surmised the function of the lymphatics. Aselli, an anatomist and surgeon in Milan, performed a vivisection on a dog to demonstrate its nerves to colleagues. In addition, he sought to analyze the function of the diaphragm and swept the bowel downwards. To his amazement, he observed multiple white chords over the bowel and mesentery, which he opened and reported white fluid emanating from them. The next day he had wanted to reproduce the same dissection on another dog, but to his disappointment, he did not observe the white chords. He hypothesized that their presence was related to the time of feeding, and he reperformed the experiment on a recently fed dog, and to his delight, he observed the white chords again. He named these chords venae albae et lactae and suggested that chyle was absorbed from the intestines via these chords and transported to the liver because, at the time, it was erroneously considered the origin of blood.
Pecquet (1622–1674), an anatomist at Montpellier, made the next major discovery. In 1651, he published a report on experiments he had performed as a medical student on the thoracic cavities of dogs. He noted that white fluid emanated from a stump in the superior vena cava and thought this was an abscess. However, upon pressing the abdomen, the flow increased. He went on to describe the cisterna chili, the thoracic duct, and its insertion point at the confluence of the left jugular and subclavian veins.
In 1643, Bartholin confirmed Pecquet’s findings in humans. Moreover, he demonstrated that these vessels also flow from the liver, and are, thus, not confined to the intestines. He published Vasa Lymphatica , and his name for these newly discovered vessels has been retained to this day. In 1935, Drinker demonstrated that one of the most important functions of the lymphatic system was to absorb protein from the interstitium and return it to the venous system to maintain blood volume. These aforementioned persons made the key discoveries regarding the anatomy and function of the lymphatic system.
Embryology of the lymphatic system
Although a large body of work exists on the function of the lymphatic system, its genesis is not completely elucidated. However, it is recognized that initially the primordial lymphatic system derives from 2 paired and 2 unpaired endothelial sacs that arise as outgrowths from the primitive venous system during the fifth gestational week. First, 2 paired sacs appear at the junction of the subclavian and jugular veins; these outpouchings create the lymphatic sacs of the upper limbs ( Fig. 1 ). Subsequently, 2 more unpaired sacs arise, one in the retroperitoneal space at the root of the mesentery and another dorsal to the mesentery; the latter becomes the cisterna chili. Finally, 2 paired sacs, the iliac lymphatics, emanate from the junction between the sciatic and the femoral veins. Several endothelial channels link these primordial lymphatic sacs by the ninth gestational week to form a complex network. Around this time, mesenchymal cells invade the lymphatic sacs; once encapsulated, the lymphatic sacs become true lymph nodes. True lymphatic function derives from the invasion of the sacs by lymphocytes.
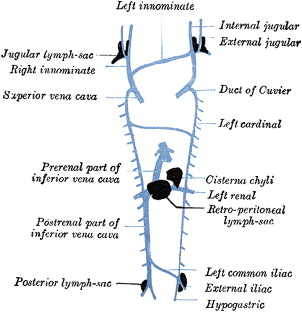
The upper and the lower lymphatic system are connected by the thoracic duct. Originally, the jugular lymphatic sacs are connected to the cisterna chili by 2 thoracic ducts that have an anastomotic channel between them. Regression occurs such that the final thoracic duct is formed from the caudal right duct, the anastomotic channel, and the proximal left duct.
Embryology of the lymphatic system
Although a large body of work exists on the function of the lymphatic system, its genesis is not completely elucidated. However, it is recognized that initially the primordial lymphatic system derives from 2 paired and 2 unpaired endothelial sacs that arise as outgrowths from the primitive venous system during the fifth gestational week. First, 2 paired sacs appear at the junction of the subclavian and jugular veins; these outpouchings create the lymphatic sacs of the upper limbs ( Fig. 1 ). Subsequently, 2 more unpaired sacs arise, one in the retroperitoneal space at the root of the mesentery and another dorsal to the mesentery; the latter becomes the cisterna chili. Finally, 2 paired sacs, the iliac lymphatics, emanate from the junction between the sciatic and the femoral veins. Several endothelial channels link these primordial lymphatic sacs by the ninth gestational week to form a complex network. Around this time, mesenchymal cells invade the lymphatic sacs; once encapsulated, the lymphatic sacs become true lymph nodes. True lymphatic function derives from the invasion of the sacs by lymphocytes.
The upper and the lower lymphatic system are connected by the thoracic duct. Originally, the jugular lymphatic sacs are connected to the cisterna chili by 2 thoracic ducts that have an anastomotic channel between them. Regression occurs such that the final thoracic duct is formed from the caudal right duct, the anastomotic channel, and the proximal left duct.
History of surgical lymphadenectomy
The importance of the lymphatic system with respect to malignant spread was first recognized in breast cancer. Ambrose Pare (1510–1590), a French battlefield surgeon, was probably the first surgeon to observe that the axillary lymph nodes may be involved in breast cancer. In the same era, Michael Servetus (1511–1553), a Spanish scientist, suggested that these nodes be removed as part of a radical mastectomy. Their thoughts were largely ignored and axillary dissection was only revisited 100 years later when Henri Le Dran and Jean Petit, 2 eminent French surgeons, suggested that axillary dissection may have a therapeutic benefit.
In 1844, Joseph Pancoast authored the first published report describing lymphadenectomy (LND), which described axillary dissection. However, the technique only become widely accepted and disseminated through the surgical community when, in 1895, Halsted published his report on axillary dissection ( Fig. 2 ). He advocated a systematic and sequential dissection and argued for the removal of the nodes en bloc with the primary cancer. Rapidly, surgeons from other organ systems recognized the importance of LND in cancer surgery. Moynihan, a contemporary of Halsted, referring to rectal surgery, exclaimed: “the surgery of malignant disease is not the surgery of organs; it is the anatomy of the lymphatic system.” In fact, the most accepted theory of cancer dissemination at the time was the lymphatic dominant spread postulated by the Handley and popularized by Halsted, which stated that cancer spread to lymph nodes first and secondarily via lymphatic channels from the lymph nodes to visceral organs. It was thought that the cancer cells became trapped in the lymph nodes, and there was a window period within which distant metastases could be avoided despite nodal involvement. This theory was challenged as early as 1913 when Tyzzer reported on several patients with distant metastases in the absence of regional nodal involvement. The theory was further challenged by Fisher who reported in 1964 that LND did not decrease the appearance of distant metastases and hypothesized that the cancer was a systemic disease from the outset whereby cancer cells escape into both the systemic circulation and lymphatic system in all patients. It is now apparent that cancer spreads via both pathways. However, it is recognized that the Halstedian principle is sound in asserting that cancer is a local disease that becomes systemic if left untreated and, thus, cure is possible if the disease remains local or locoregional.
The anatomy and history of lymphadenectomy for specific urologic malignancies
Kidney
The lymphatic drainage of the 2 kidneys varies. The left kidney drains primarily into the para-aortic nodes, which are bordered cranially by the diaphragm and caudally by the inferior mesenteric artery. Rarely, the left kidney may drain into the retrocrural nodes or directly into the thoracic duct. The right kidney drains primarily into the para-caval, precaval, and interaortocaval nodes ( Fig. 3 ).
Classically, renal cancer presented with a triad of flank pain, hematuria, and a palpable flank mass. However, with the advent of modern imaging, less than 10% of contemporary patients will present with this constellation. Moreover, less than 5% of contemporary patients will exhibit lymphadenopathy on preoperative imaging. However, this was not the case in 1948 when Mortensen first described the modern radical nephrectomy, which included removing all contents within the Gerota fascia, including the kidney, adrenal, and perirenal fat. It was not until 1963 that Robson reported that 22.5% of patients in his case series had renal-hilar lymph node involvement and, thus, he suggested performing a regional LND along with radical nephrectomy. When he updated his series in 1969, he demonstrated a significant survival advantage in patients who had received a regional LND, even after adjustment for tumor size. The template for renal LND has not really changed since that time, and although several retrospective series have demonstrated survival benefit in those undergoing LND, the only randomized trial published did not corroborate those earlier findings. Moreover, since the advent of laparoscopic nephrectomy and because of its extensive use, the performance of a renal LND has decreased substantially.
Bladder
The lymphatic drainage of the bladder is complex and dependent on bladder location. The wall itself has lymphatics in the submucosa, detrusor, and the perivesical fat. The nodes of the base, neck, and trigone terminate in the internal, external, and sacral nodes. The anterior wall nodes primarily feed the external iliac. The posterior wall drains into the hypogastric nodes. The second echelon of drainage is the common iliac and distal aortocaval nodes. Regional lymph node mapping has identified the external iliac (65%) and obturator (74%) to be the 2 most commonly involved sites of metastases.
At the time of cystectomy for muscle invasive disease, nearly 25% of patients will have pathologic lymph node metastases. Although LND has become a standard component of the contemporary radical cystectomy, this was not always the case. Despite the Halstedian view that primary cancers with solely lymph node involvement were amenable to surgical treatment, the prevailing view in the bladder cancer community flanking the turn of the century was that grossly palpable nodes indicated uniformly fatal disease. The first study to challenge this dogma was reported by Colston and Leadbetter in 1936, who performed autopsies on 98 patients with bladder cancer. They observed a significant number of patients that had solely nodal metastases and, therefore, hypothesized that these patients were potentially curable with surgery. Ten years later, Jewett and Strong confirmed these findings in 1946 in a similar autopsy study that included patients with extravesical disease. Moreover, they demonstrated a correlation between the depth of the bladder tumor and nodal metastatic burden. Their findings became the basis for the Marshall-Jewett system, the first staging system for bladder cancer.
It was not until 1950 that data emerged suggesting a clinical benefit from pelvic LND. Colby and Kerr reported on 2 patients with nodal disease that experienced long-term survival. Moreover, the investigators reported a high number of local recurrences, and, thus, suggested 2 technical improvements: wide bladder excision and inclusion of a pelvic LND (PLND), both of which have stood the test of time. That same year (1950), Leadbetter and Cooper were the first to describe a detailed technical template for performing LND for bladder cancer. Based on the established bladder lymphatic drainage patterns, their limits were the distal aorta proximally, the genitofemoral nerve laterally, and both the circumflex iliac vein and the node of Cloquet distally. This template essentially represents a contemporary extended pelvic LND (ePLND) ( Fig. 4 ). The survival benefit of ePLND for patients with nodal disease was later validated by reports from both Whitmore and Leadbetter himself describing nodal positive patients with long-term survival. Moreover, the latter study concluded that ePLND was not associated with increased morbidity. In 1982, Skinner published his first series on the contemporary use of an ePLND and demonstrated 30% long-term survival in patients who were node positive.
The utility of the template described by Leadbetter and championed by Skinner remained unchallenged for almost 40 years. However, in 1987, a study by Wishnow suggested that LND above the bifurcation could be safely avoided proximal to the iliac bifurcation in those patients with no gross pelvic nodal disease. This limited template became known as the standard or limited PLND even though his results were based on only 18 patients who were node positive (see Fig. 3 ). More recent data from Stein, at the University of Southern California, and other large cohort studies refute this hypothesis, and an ePLND is the template that has emerged as the standard of care in centers of excellence today.
Prostate
The first radical prostatectomy was described by Kucher in 1866 and was completed via the perineal approach. However, the technique only became widely accepted after Hugh Hampton Young reported on his initial series of patients in 1905, with a more anatomic and practical description. In 1945, Millin described the retropubic prostatectomy for benign disease, which was later modified by Whitmore to treat malignant disease and, thus, included LND in some patients. However, the procedure was not widely practiced because of the significant morbidity associated with the procedure. Thus, the transperineal approach, precluding formal PLND, became the procedure of choice for clinically localized prostate malignancy until reports in the late 1970s suggested that PLND had increased sensitivity for detecting occult lymph node involvement (LNI) compared with preoperative imaging techniques and, thus, added important staging information. Around the same time, in 1982, Walsh described the anatomic retropubic radical prostatectomy (RRP), which benefited from enhanced understanding of the dorsal venous complex, and, thus, blood loss and consequent morbidity could be reduced. Moreover, reports from Walsh detailing enhanced understanding of the urinary sphincter and the neurovascular bundles provided for superior continence and sexual function outcomes. Thus, RRP with concomitant PLND became a standard of care for all patients with locally resectable prostate cancer until the Partin tables were published, which accurately quantified the risk of occult lymph node involvement.
With the advancement in urologic laparoscopy around the early 1990s, laparoscopic pelvic LND developed into a useful tool for detecting occult lymph node metastases in patients in whom external beam radiotherapy was planned or in patients who were undergoing perineal prostatectomy. Formal pelvic LND is still widely practiced in men undergoing both open and laparoscopic RRP and remains the most accurate modality for identifying pelvic metastases.
Although LND was thought to confer staging information and enhance prognostication, the therapeutic benefit in men with prostate cancer remained controversial even in patients with clinically unapparent LNI (stage D1). In fact, Walsh surmised in 1987 that: “It is unlikely that radical surgery will cure any patient with Stage D1 adenocarcinoma of the prostate.” However, in that same year, data emerged supporting a therapeutic benefit for PLND. Golimbu and colleagues, in a study of 42 patients, demonstrated enhanced survival in patients with minimal LNI detected after PLND compared with a matched cohort that had not undergone PLND. Although no randomized controlled trials have been performed to support curative PLND, multiple case series support moderate cancer-specific survival outcomes for patients with LNI. Moreover, in 2006, Masterson and Joslyn demonstrated improved survival outcomes in patients that had undergone ePLND compared with standard PLND in patients who were node negative and node positive, respectively.
Standard PLND for prostate cancer, which is now considered a limited dissection, extends proximally from the iliac bifurcation to the circumflex iliac vein distally and medially from the obturator nerve to the external iliac vein laterally ( Fig. 5 ). In 2002, Heidenreich reported that performing an ePLND, including external, internal, and common iliac; obturator; and presacral, increased the detection of lymph node metastases from 12% to 27% (see Fig. 5 ). Moreover, there were patients that had solely positive hypogastric nodes, which laid doubt to the dogma that the obturator and external iliac nodes were the primary prostate drainage sites. Others have reported similar findings. Most recently, Mattei and colleagues, using intraprostatic technetium injection and fused single-photon emission computed tomography (SPECT) combined with either computed tomography (CT) or magnetic resonance imaging (MRI), demonstrated that more than 35% of the prostate landing zones are proximal to the iliac bifurcation and that more than 20% of the primary echelon nodes may be proximal to the ureteric crossing of the iliac vessels ( Fig. 6 ).










