Radiation therapy (RT) represents an important therapeutic component in the management of genitourinary (GU) malignancies. RT is used to treat patients with proven involvement of the regional lymph nodes or delivered electively to patients at risk for occult regional lymph node metastases. Advances in treatment planning and delivery of various types of RT provide the technology to precisely plan, target, and deliver RT with the goal of optimizing the radiation dose to the target while sparing normal tissue. This article provides an overview of the modalities, indications, and techniques of RT for treatment of the lymphatic basins in GU malignancies.
Radiation therapy (RT) represents an important therapeutic component in the management of many genitourinary (GU) malignancies. RT has been used to treat patients with proven involvement of the regional lymph nodes or delivered electively to patients at risk for occult regional lymph node metastases. Lymphatic basins at risk in GU malignancies include the presacral, external iliac, internal iliac, common iliac, para-aortic, and inguinal lymph node chains. The specific nodal basin and degree of risk is dependent on the primary cancer site, local extent of disease, and other prognostic factors. An overview of the modalities, indications, and techniques of RT for treatment of the lymphatic basins in GU malignancies is reviewed here.
Basic principles of radiation therapy
External Beam Radiation Therapy
External beam radiation therapy (EBRT) is a form of RT delivered from a source outside of the body. EBRT can be delivered in the form of photons (x-rays), charged particles (electrons or protons), neutrons, or heavy ions. Photons are the most common modality for delivery of nodal radiation in GU malignancies based on widespread availability, depth of tissue penetration, and dose-distribution properties.
The implementation of computed tomography (CT)-based EBRT planning and sophisticated dose-modeling software has revolutionized the field of radiation oncology. Conventional EBRT (2DXRT) is planned using 2-dimensional radiographs. Soft tissue structures are poorly visualized on these images; therefore, beam arrangements are designed in reference to bone landmarks to target an area of interest for treatment. This technique is well established, efficient, and reliable; however, 2DXRT lacks the dose modeling and conformity capabilities of more modern treatment-planning techniques. With the development of CT, 3-dimensional conformal radiation therapy (3DCRT) has become the most widely used method of treatment planning and delivery ( Fig. 1 ). CT simulation provides the radiation oncologist with the ability to reconstruct the tumor and normal structures in 3 dimensions. In addition, multiple beam arrangements conform to the shape of the target area with consideration of individual patient anatomy and normal tissue tolerance of radiation dose.
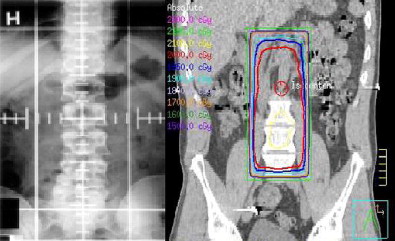
The treatment-planning process begins with a CT scan of the patient in a reproducible position to create a virtual simulation of 3-dimensional daily treatment. With the aid of computer software and treatment-planning stations, the radiation oncologist selects the beam arrangement that optimizes dose distribution to the area at risk while minimizing the dose to surrounding normal tissue, thereby enhancing the therapeutic ratio.
Intensity-Modulated Radiation Therapy
Intensity-modulated radiation therapy (IMRT) is a specialized form of 3DCRT. Compared with most other treatment techniques, IMRT can achieve a more conformal radiation plan than with standard 3DCRT, thus further reducing dose and toxicity to normal tissue. The advantages of IMRT are particularly evident when the target volumes have complex shapes or concave regions. IMRT treatment planning is similar to 3DCRT; however, whereas 3DCRT delivers a uniform dose from each RT beam, IMRT has the added capability of varying the RT intensity within each beam. This allows the radiation oncologist to define the clinical target volume and set dose constraints to surrounding normal structures. Computer algorithms are used to modulate the intensity of the radiation beams to optimize the treatment plan ( Fig. 2 ). Furthermore, varying the dose administered within each beam enables IMRT to simultaneously treat multiple areas within the target to different dose levels, thus providing a simultaneous integrated boost (SIB). Tight conformity of dose distributions to target structures allows IMRT to better preserve surrounding normal structures within the pelvis/abdomen from high-dose radiation, thereby limiting treatment-related toxicity. For GU malignancies, IMRT allows for dose escalation to sites of gross disease or nodal involvement while reducing the radiation dose to the small bowel, rectum, and bladder. Patient immobilization, positioning, and limitation of organ motion are essential for daily reproducibility when delivering highly conformal treatments. IMRT offers potential advantages in the management of specific GU malignancies; these are addressed later in this article.

Charged Particles
Electrons and protons are charged particles that penetrate a certain distance into the body before depositing their energy. Electrons penetrate a short distance into tissue; therefore, electrons can be useful for treatment of superficial targets, such as the inguinal lymph nodes. Protons can penetrate much deeper into tissue than electrons. Protons also possess a more predictable range of energy deposition. Proton therapy provides an ability to more precisely localize the radiation dose at a depth in tissue similar to that achieved with photon therapy. These properties have made proton therapy an area of interest for treatment of regional lymphatics in GU and other pelvic malignancies.
Target Volume and Dose
The dose of radiation required to treat solid tumors with definitive EBRT ranges from approximately 60 to 80 Gy. For sterilization of microscopic disease, as is often the case for elective lymph node coverage, the dose ranges from 45 to 60 Gy. The total dose is typically fractionated or spread out over time. Standard EBRT is delivered in once-daily fractions, typically 1.8 to 2.0 Gy, 5 days a week, for several weeks until the total prescribed dose is achieved. Fractionation decreases the amount of toxicity to healthy tissues by exploiting their capacity for sublethal damage repair and repopulation compared with malignant tissue. Throughout the course of treatment, tumor cells undergo reoxygenation and reassortment into more sensitive phases of the cell cycle. Both of these processes increase their sensitivity to fractionated regimens.
Treatment-Related Toxicity
The incidence and severity of RT side effects depend on multiple factors, including the site and volume of tissue exposed, radiation dose, type of radiation, and fractionation pattern. Other modifying factors can influence RT-related toxicity, such as previous surgery, concomitant chemotherapy, and comorbid medical conditions. Acute and subacute side effects occur during or within the first several months following completion of treatment. Acute and subacute side effects associated with treatment of GU cancer regional lymph nodes include injury to the skin and mucosal surfaces, fatigue, and diarrhea. Late effects are side effects occurring several months or years after RT. These effects can include fibrosis/bowel obstruction, stricture, fistula, radiation enteritis/proctitis, intestinal malabsorption, lymphedema, or secondary malignancies. The primary organ of concern when administering RT to regional lymph nodes for patients with GU malignancies is the small bowel. Although normal small bowel motion helps to reduce exposure to pelvic radiation, it can be at risk for late toxicity if excessive bowel is within the radiation field. An example of this is when adhesions from previous surgeries immobilize the small bowel within the pelvis. As part of the recent Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) project, limiting the small bowel volume receiving more than 45 Gy to less than 195 mL substantially reduces the risk of acute grade 3 or greater gastrointestinal toxicity. These dose/volume constraints are likely to translate to reduced late toxicity as well.
Site-specific discussion
Prostate Cancer
Elective coverage of regional nodes
The pathologic status of the regional lymph nodes is typically unknown in men with clinically localized prostate cancer managed with RT. Risk of pelvic nodal metastasis for prostate cancer was initially established through pelvic lymph node dissection: generally, limited pelvic lymphadenectomies. Optimal RT for prostate cancer requires the accurate assessment of risk factors predicting pathologic stage and prognosis. The current standard is to characterize the risk of biochemical failure and prostate cancer–specific mortality according to a patient’s clinical (TNM) stage, Gleason grade, and serum prostate-specific antigen (PSA) level. Certain nomograms, equations, and tables have been developed by combining prognostic factors to predict pathologic stage, extracapsular spread, seminal vesicle involvement, and pelvic nodal metastasis. Roach and colleagues derived a simple equation to estimate the risk of pathologic pelvic lymph node involvement: positive lymph node % = (2/3) PSA × ([Gleason score – 6] × 10). Recent application of the Roach formula to population datasets suggests this simple equation may overestimate the true risk of pelvic lymph node disease in contemporary patients. Although advanced T-stage tumors are seen less frequently in the era of PSA screening, clinical T3–T4 disease also predicts a high risk for pelvic nodal metastasis and is notably absent from the Roach formula. Regardless, the Roach formula is one of the many tools commonly used to estimate the likelihood of regional lymph node involvement and thus select patients for whole-pelvis RT (WPRT).
Patients with a less than 10% risk of lymph node metastases do not routinely require radiographic staging of the regional lymph nodes before curative treatment ; however, improved methods to identify occult disease are greatly needed for patients with intermediate or high risk of nodal metastasis. Currently, CT scan and/or magnetic resonance imaging (MRI) scans are routinely used for pelvic nodal staging of prostate cancer. The limitations of CT imaging are such that a normal CT does not rule out the presence of nodal disease. Although MRI is superior to CT scan in identifying the local extent of prostate tumors, it does not appear to provide any significant benefit in identifying nodal metastasis. Both of these modalities are currently dependent on identification of lymph node enlargement. Alternative imaging techniques independent of anatomic distortion would be useful for lymph node staging. This topic is covered in the article by Chernyak and colleagues elsewhere in this issue of Urology Clinics .
Regardless of the method of detection, regional therapy to involved nodal disease or areas at high risk of lymph node involvement seems reasonable, considering sterilization of all microscopic disease is a prerequisite for cure. If WPRT is effective at sterilizing microscopic disease, then progression-free survival (PFS) and overall survival (OS) should improve in patients with significant risk for regional nodal involvement. This rationale has been validated in other adenocarcinomas in which EBRT to regional lymph nodes is routine.
The role of WPRT for men with intermediate-risk or high-risk prostate cancer remains uncertain. Some continue to advocate WPRT for men with an estimated risk of regional lymph node involvement of 15% or more. Others have recommended WPRT be restricted to the investigational setting. Two randomized trials have addressed the role of WPRT compared with prostate-only RT. Although both of these trials have failed to show an advantage of WPRT compared with prostate-only treatment, criticisms of both trials have allowed the issue of elective lymph node coverage in intermediate-risk to high-risk prostate cancer to remain unresolved.
In the Radiation Therapy Oncology Group (RTOG) 9413 trial, 1323 men with clinically localized prostate cancer and an estimated risk of nodal metastases of 15% or more were randomly assigned to WPRT (50.4 Gy to the pelvis and 70.2 Gy to the prostate using conventional techniques) or prostate-only RT (70.2 Gy). Patients were further randomized, using a 2 × 2 factorial design, to neoadjuvant plus concurrent androgen deprivation therapy (ADT) administered for 2 months before and during RT, or adjuvant ADT given for the same length of time, but starting after the completion of RT. Most enrolled men had intermediate-risk or high-risk disease. The initial report of this trial suggested WPRT plus neoadjuvant plus concurrent ADT improved PFS compared with any of the 3 other treatment combinations. The long-term analysis of this study showed no significant differences in PFS or OS when men treated with WPRT were compared with prostate-only RT. Within the group that received WPRT, there was a trend toward better PFS and OS with neoadjuvant plus concurrent as compared with adjuvant-only ADT. The study was insufficiently powered to show a statistically significant difference between these 2 treatment arms. An unplanned subset analysis of RTOG 9413 was performed to determine whether RT field size significantly influenced PFS. The analysis was limited to patients receiving neoadjuvant and concurrent hormone therapy to control for the interaction of ADT timing. The median and 7-year PFS improved with increased field size. However, late grade 3 gastrointestinal complications correlated with increasing field size as well. A complicated and unanticipated interaction between the timing of hormone therapy and radiation field size complicates interpretation of RTOG 9413. Furthermore, the use of WPRT with ADT in predecessor trials allows proponents of this approach to suggest WPRT should remain the standard in the setting of equivocal results.
The results of a second trial support the long-term results of RTOG 9413. In a trial by the Genitourinary Study Group (Groupe d’Etude des Tumeurs Uro-Génitales), GETUG-01, 446 men were randomly assigned to WPRT or prostate-only RT. ADT was allowed for high-risk patients at the treating physician’s discretion. Use of ADT was balanced between the 2 groups. At a median follow-up of 42 months, the 5-year PFS and OS were not significantly different between the 2 treatments. This trial has been criticized for including men with more favorable disease (more than 50% of men with estimated risk of pelvic lymph node involvement <15%), relatively low RT dose delivery, smaller RT field size for WPRT, and using an antiquated definition of biochemical failure. Despite their limitations, there are now 2 randomized trials showing no significant differences in PFS or OS between prostate-only and elective pelvic lymph node treatment for men with clinically localized prostate cancer. Further study and longer follow-up of these issues are warranted before a standard of care can be recommended. Based on the available results, WPRT can be considered in cases where the risk of lymph node involvement is greater than 15%; however, WPRT should be used judiciously considering the potential for worse gastrointestinal toxicity with larger field size.
Postoperative RT
The use of RT alone for men with node-positive disease has been associated with high rates of local and distant recurrence. RT alone has been used primarily for palliation for men with known lymph node involvement. The natural history of node-positive prostate cancer treated with RT alone is best illustrated by a subset analysis of RTOG 7506. In a total of 90 men with regional node-positive prostate cancer, the 10-year survival rate was 29%. Only 5 patients were progression free at 10 years. Three of the 5 patients without progression were not assessed by PSA.
There are only limited data on combining radical prostatectomy with postoperative RT in men with lymph node–positive prostate cancer. There are several nonrandomized series suggesting a benefit of postoperative adjuvant RT in select men with pathologically positive regional lymph nodes. Men with a lower burden of regional lymphatic disease may be the population of patients who benefit most from postoperative RT. Briganti and colleagues conducted a recent matched-pair analysis of 703 consecutively treated patients with documented lymph node metastases (pN+). Comparisons were made between patients treated with adjuvant ADT alone versus adjuvant ADT plus WPRT. Patients were matched for age at surgery, pathologic T stage, Gleason score, number of nodes removed, surgical margin status, and length of follow-up. Patients treated with adjuvant WPRT plus ADT had significantly higher 10-year prostate cancer–specific survival (86% vs 70%; P = .004) and OS (74% vs 55%; P <.001) compared with patients treated with ADT alone. Similar improvements were seen when patients were stratified according to the extent of nodal invasion (≤2 vs >2 positive nodes; P = .006). These results reinforce the benefits of a multimodality approach in the treatment of node-positive prostate cancer.
The efficacy of RT as a primary treatment modality for localized prostate cancer suggests a role for postoperative RT in men with node-negative disease who have a high likelihood of residual cancer in either the prostate bed or regional lymph nodes. To realize a benefit using this approach, certain presumptions must be true. First, any residual disease must be confined to a definable treatment volume. Second, the toxicity from additional local therapy must be low and manageable. RT can be integrated into the postoperative management of prostate cancer either by delivery soon after surgery to those at high risk for relapse with an undetectable PSA (adjuvant RT) or delayed delivery until evidence of PSA relapse (salvage RT). The former approach has the advantage of treating a potentially lower disease burden, although unnecessarily treating a preponderance of men who were not actually destined for PSA relapse.
Historically, postoperative RT for men with node-negative disease has been limited to the prostate bed and has not included elective treatment of the pelvic lymph nodes. There are 3 published randomized controlled trials comparing adjuvant RT with no planned additional treatment for patients at high risk of local failure following prostatectomy. All of these trials showed that adjuvant RT is effective at reducing progression with indications for adjuvant therapy including pT3 disease or positive surgical margin. The target volume in all of these trials included the prostatic fossa without specific targeting of the regional lymphatics. RTOG 96-01 compared adjuvant RT with or without an antiandrogen. This trial also did not allow for inclusion of the regional lymph nodes as a radiation target. In part because of the lack of clarity regarding elective lymph node treatment in the intact prostate setting, the RTOG initiated a trial to evaluate elective regional lymphatic coverage and the use of ADT in men with a rising PSA following prostatectomy. RTOG 0534 includes 3 arms: prostate bed–only RT; prostate bed RT plus ADT; and WPRT plus ADT. RTOG 0534 is currently in the active phase and results are not anticipated for several years.
Bladder Cancer
The implementation of effective radiosensitizing chemotherapy agents has allowed chemo-radiation to become an integral component of a bladder-conserving approach for select patients with muscle-invasive bladder cancer. In this setting, EBRT is delivered using a conventional 4-field treatment technique targeting the bladder and first-echelon lymph nodes. The lymphatic drainage of the urinary bladder includes the external iliac, obturator, and other internal iliac chain lymph nodes situated along the pelvic sidewall medial to the acetabulum. The superior border of the pelvic field is placed at the bifurcation of the common iliac arteries (approximately mid-sacroiliac joint) creating a “mini-pelvis” field ( Fig. 3 ). After a dose of approximately 40 to 45 Gy is delivered to the pelvis with concurrent chemotherapy, the response to treatment is assessed through cystoscopy with directed biopsies and urine cytology. If a complete response is obtained in the primary tumor, an additional 20 to 28 Gy is delivered to the bladder/ primary tumor before transurethral resection of bladder tumor. Improvements in the delivery of radiation therapy with improved conformal techniques, including IMRT, proton beam therapy, and the use of image guidance, will likely further improve outcomes of bladder-preserving treatments and allow conformal dose-escalation to the regional lymph nodes. Such improvements may also reduce the volume of normal tissues exposed to higher doses and thereby are likely to decrease the morbidity of therapy, especially when combined with radiosensitizing systemic agents.
Testicular Cancer
Regional lymph node RT for men with testicular cancer is primarily confined to men with early-stage seminoma and is slightly different from RT for other GU malignancies. First, the dose required to sterilize microscopic disease or gross nodal disease for seminoma is markedly lower than for other solid tumors (20–30 Gy and 30–40 Gy, respectively). Second, the retroperitoneal lymph nodes are considered first-echelon lymph nodes because of the specific lymphatic drainage of the testicles. EBRT effectively prevents relapse in most patients with clinical stage I and nonbulky stage II seminoma. Although most patients experience no serious adverse treatment effects, impaired fertility, second malignancies, and bowel effects have raised concerns about adjuvant EBRT in this setting. These concerns led to the evaluation of active surveillance and adjuvant chemotherapy as alternative treatments in men with stage I seminoma. Nevertheless, adjuvant RT remains an important option in carefully selected patients.
The technique for adjuvant infradiaphragmatic RT has evolved over the past several decades to include a smaller RT target area with less radiation dose.
Target for adjuvant radiation
Excellent results have been reported using a “dog-leg” RT field. This field includes the ipsilateral renal hilum/pelvic lymph nodes and the bilateral para-aortic nodes. Considering the PA lymph nodes are the first-echelon nodes for testicular tumors, a prospective trial by the Medical Research Council (MRC) Testicular Tumor Working Group directly compared PA-only RT to a classic “dog-leg” RT field following inguinal orchiectomy. In this trial, 478 men with stage I testicular seminoma were randomly assigned to one of the adjuvant RT treatments. The short-term side effects of RT were improved and the incidence of azospermia was significantly decreased in patients receiving PA-only RT. The number of pelvic relapses was higher in the group receiving PA-only RT, but the total number of relapses were similar. Three-year survival was equivalent (>99%). As a result, PA-only RT is considered the standard treatment for men with stage I seminoma receiving adjuvant RT. Inclusion of the ipsilateral pelvic lymph nodes may be considered when there is risk of aberrant nodal drainage based on prior history and is considered standard adjuvant therapy for men with nonbulky clinical stage II seminoma following orchiectomy.
Radiation dose
Owing to the radiosensitivity of seminoma, the required doses for sterilization of microscopic or macroscopic disease are some of the lowest used in therapeutic radiation. The MRC conducted a multinational trial where 625 men with seminoma were randomly assigned to 20 Gy delivered in 10 fractions, or 30 Gy delivered in 15 fractions following orchiectomy. There was no difference in disease-free survival (approximately 98%). Men treated with higher-dose radiation had significantly more lethargy (20% vs 5%) and inability to work (46% vs 28%) measured 1 month after treatment. These differences disappeared by 3 months after RT. Alternative dose regimens using a lower dose per fraction to a slightly higher, but biologically equivalent, dose are common in the United States (ie, 25.5 Gy delivered in daily 1.5-Gy fractions). For areas with gross nodal involvement, a smaller “boost” field is used to bring the total dose to 36 Gy.
Penile Cancer
The inguinal lymph nodes are the primary lymphatic basin at risk in men with penile cancer. Elective RT to the inguinal lymph nodes is not recommended for patients without cytologically or histologically proven lymph node metastases or in those men with microscopic lymph node metastases treated with inguinal lymph node dissection. This contention is because of the potential for treatment-related morbidity (dermatitis, impaired wound healing, lymphedema) without a clearly defined benefit of RT versus surveillance alone in this population. Inguinal RT might improve local control for men with multiple positive lymph nodes, bulky disease (>4 cm), or extranodal extension. The possible morbidity of an uncontrolled inguinal recurrence must be weighed against the risk of treatment-related toxicity. The evidence for treatment of the regional lymph nodes in patients with high-risk factors for nodal recurrence is primarily supported though small retrospective series and extrapolations from other disease sites (vulvar and head/neck squamous cell carcinoma) where postoperative treatment of the regional lymph nodes in select patients seems to provide a therapeutic gain. Prospective trials are needed to better define the role of regional lymph node radiation in men with penile cancer who have high-risk features for recurrence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







