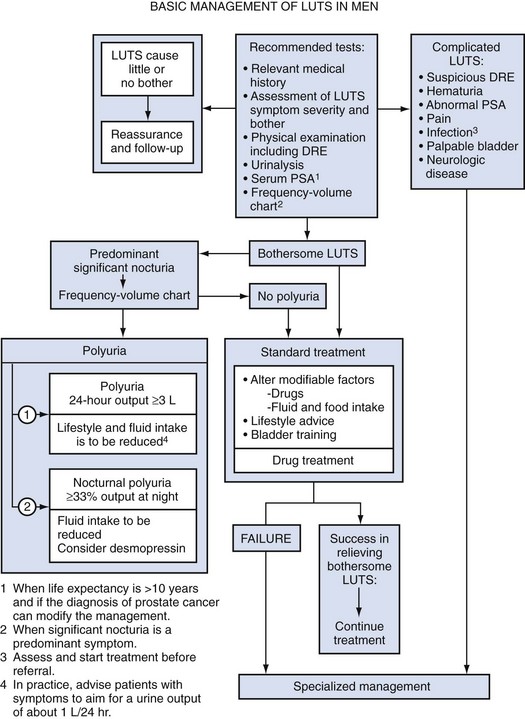
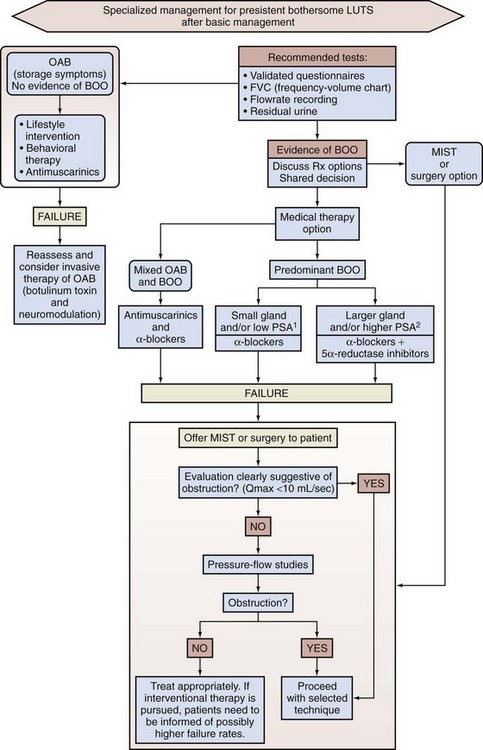
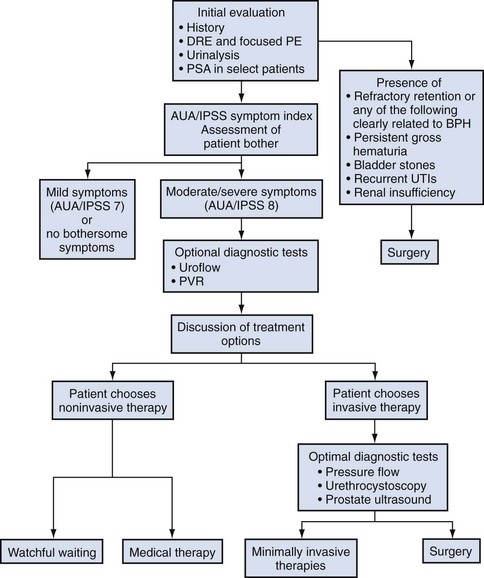
Thomas Anthony McNicholas, MBBS, FRCS, FEBU, Roger Sinclair Kirby, MD, MA, FRCS, Herbert Lepor, MD The term benign prostatic hyperplasia (BPH) has very different connotations to the pathologist, radiologist, urodynamicist, practicing urologist, and patient. BPH to the pathologist is a microscopic diagnosis characterized by cellular proliferation of the stromal and epithelial elements of the prostate. The radiologist confirms the diagnosis of BPH on the basis of an enlarged prostate either with ultrasound or three-dimensional diagnostic imaging studies of the male pelvis (Haas and Resnick, 2000). The hallmark of BPH to the urodynamicist is the synchronous observation of elevated voiding pressure and a low urinary flow rate in the absence of other disease processes that cause bladder outlet obstruction (BOO) (Nitti, 2000). BPH to the practicing urologist represents a constellation of signs and symptoms that develop in the male population in association with aging and prostatic enlargement presumably caused by BOO (Shapiro and Lepor, 1995), together with ultrasound imaging. The patient is typically concerned about the impact of BPH on quality of life rather than about the presence of cellular proliferation, prostatic enlargement, or elevated voiding pressures. Because of the diverse connotations of the term, it seems sensible to define BPH as microscopic BPH, macroscopic BPH, or clinical BPH. Microscopic BPH represents histologic evidence of cellular proliferation of the prostate. Macroscopic BPH refers to enlargement of the prostate resulting from microscopic BPH. Clinical BPH represents the lower urinary tract symptoms (LUTS), bladder dysfunction, hematuria, and urinary tract infection (UTI) resulting from macroscopic BPH. Abrams (1994) has suggested using the more clinically descriptive terms benign prostatic enlargement (BPE), bladder outlet obstruction (BOO), and lower urinary tract symptoms to replace BPH, but the time-honored, although limited, original terminology persists. Microscopic BPH describes a proliferative process of the stromal and epithelial elements of the prostate (Bartsch et al, 1979). The proliferative process originates in the transition zone and the periurethral glands (McNeal, 1978). It is rarely identified in men younger than 40 years of age (Berry et al, 1984). The autopsy incidence of BPH is age dependent, the proliferative process being present in approximately 70% and 90% of men in their seventh and ninth decades of life, respectively. The development of microscopic BPH requires aging and the testes as the source of androgens (Walsh, 1984). Androgens play a passive role in the proliferative process. The specific molecular events that initiate and promote microscopic BPH have yet to be identified and characterized. Growth factors, such as epidermal growth factor (EGF), are involved through autocrine and paracrine stromal-epithelial interactions (Steiner, 2000). Macroscopic BPH describes an “enlarged” prostate. Digital rectal examination (DRE) provides a relatively crude estimate of prostate size when compared with measurements using transrectal ultrasonography or magnetic resonance imaging (Roehrborn et al, 1997). Knowledge of prostate size may be clinically relevant in terms of selecting appropriate medical or surgical therapy. A strong correlation exists between serum prostate-specific antigen (PSA) levels and prostate volume (Roehrborn et al, 1999), and, as a consequence, in the absence of adenocarcinoma, the PSA value may be used as a surrogate for prostate volume (Roehrborn et al, 2001). The transition zone (inner gland) accounts for the majority of BPH tissue. The transition zone volume can be quantified using transrectal ultrasonography (Lepor et al, 1994) or magnetic resonance imaging (Tempany et al, 1993). There is no consensus regarding the extent of enlargement required to establish the diagnosis of macroscopic BPH; however, prostate volume of approximately 20 mL may be regarded as normal (Garraway et al, 1991) before BPH develops, and volumes of 100 mL or greater are encountered clinically. The clinical manifestations of BPH include LUTS, poor bladder emptying, urinary retention, an overactive bladder, UTI, hematuria, and renal insufficiency (Jepsen and Bruskewitz, 2000). Historically, the pathophysiology of clinical BPH was attributed to BOO secondary to macroscopic enlargement of the prostate gland (Lepor, 2000). This hypothesis was supported by epidemiologic data suggesting that the prevalence of microscopic BPH, macroscopic BPH, and clinical BPH is age dependent and therefore causally related (Isaacs and Coffey, 1989). This rather oversimplistic concept of the pathophysiology of BPH has been challenged by more recent reports demonstrating only weak relationships between prostate size, severity of BOO, and severity of symptoms (Barry et al, 1993; Bosch et al, 1995; Girman et al, 1995; Yalla et al, 1995). However, there are numerous epidemiologic data to confirm that BPH is a slowly progressive disease and that men with a larger prostate (or higher PSA) are at significantly greater risk of LUTS, impaired quality of life, and complications such as acute urinary retention (AUR) (Roehrborn et al, 2001) (see Chapter 91). The complex of symptoms now commonly referred to as “LUTS” is not specific for BPH. Aging men with a variety of lower urinary tract pathologic processes may exhibit similar, if not identical, symptoms. The initial diagnostic challenge in these patients is to establish that the symptoms are, in fact, a result of BPH. This is the primary focus of initial evaluation and diagnostic testing. Fortunately, nonprostatic causes of symptoms can be excluded in a significant majority of patients on the basis of history, physical examination, and urinalysis. Additional diagnostic testing is necessary in patients in whom the diagnosis is still unclear after initial evaluation. These tests may also have a modest (but still unproven) value in predicting the response to treatment. The following recommendations concerning the initial evaluation of men presenting with LUTS reflect the consensus opinion for several independent groups. The American Urological Association (AUA) BPH guidelines were presented in 1994 and 2003, were updated in 2010 (McVary et al, 2010), and are available at www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=bph. The European Association of Urology (EAU) has published guidelines on “Assessment, Therapy and Follow-Up of Men with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Obstruction (BPH Guidelines)” (Madersbacher et al, 2004), and in 2009 a Sixth International Consensus document was published (Abrams et al, 2009). Algorithms for the management of LUTS/BPH are shown in Figures 92-1 to 92-3. (From Abrams P, Chapple C, Khoury S, et al. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2009;181[4]:1779–87.) (From Abrams P, Chapple C, Khoury S, et al. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2009;181[4]:1779–87.) (From Kaplan SA. Update on the American Urological Association guidelines for the treatment of benign prostatic hyperplasia. Rev Urol 2006;8[Suppl. 4]:S10–7.) DRE establishes the approximate size of the prostate gland. Estimation of prostate size is important to select the most appropriate pharmacologic or technical approach. DRE provides a sufficiently accurate measurement in most cases. The size of the prostate is not critical in deciding whether active treatment is required. Prostate size does not correlate precisely with symptom severity, degree of urodynamic obstruction, or treatment outcomes (Roehrborn et al, 1986; Simonsen et al, 1987). If a more accurate measurement of prostate volume is needed to determine whether to perform open prostatectomy rather than transurethral resection of the prostate (TURP), or some other procedure such as laser vaporization, ultrasonography (transabdominal or transrectal) is more accurate than cystourethroscopy. It is now established that a larger gland, and consequently a higher PSA, is associated with a greater risk of BPH progression (Roehrborn et al, 2001). The positive predictive value of urinalysis for cancer or other urologic diseases is around 4% to 26%, depending on the patients screened and the rigor of follow-up studies (Mohr et al, 1986, 1987; Messing et al, 1987). Urine cytology should always be requested in men with severe irritable symptoms and dysuria, especially if they have a smoking history. Carcinoma in-situ of the bladder is a diagnosis that may have serious consequences if overlooked. Although the measurement of the serum creatinine concentration was recommended in the initial evaluation of all patients with symptoms of LUTS to exclude renal insufficiency caused by the presence of obstructive uropathy (McConnell et al, 1994; Denis et al, 1998), at the Fifth International Consultation on BPH it was suggested that serum creatinine determination should be optional or secondary. The AUA guidelines on BPH and the Sixth International Consensus report no longer recommend routine creatinine measurement in the standard patient. However, it is well established that BPH patients with renal insufficiency have increased risk for postoperative complications. The risk is 25% for patients with renal insufficiency, compared with 17% for patients without the condition (Mebust et al, 1989). Moreover, the mortality increases up to sixfold for BPH patients treated surgically if they have renal insufficiency (Holtgrewe and Valk, 1962; Melchior et al, 1974). Of 6102 patients evaluated in 25 studies by intravenous urography (IVU) before prostate surgery, 7.6% had evidence of hydronephrosis (McConnell et al, 1994). Of these patients, 33.6% had associated renal insufficiency. Elevated serum creatinine levels in a patient with BPH is an indication for imaging studies (usually ultrasonography) to evaluate the upper urinary tract. In a retrospective analysis of 345 patients who had undergone prostatectomy, 1.7% (n = 6) had occult and progressive renal damage (Mukamel et al, 1979). These patients had minimal or no urinary symptoms and presumably fit the category of patients with “silent prostatism.” Measurement of the serum creatinine concentration is one means to identify such “at risk” patients. Prostate cancer can lead to LUTS by producing bladder outflow obstruction similar to BPH. Moreover, localized prostate cancer commonly coexists with BPH. In most men with a 10-year or longer life span the knowledge of concomitant prostate cancer may well alter management of the BPH component. The detection of a large nodular prostate cancer on DRE would no doubt alter therapy; however, the “early detection” of small volume prostate cancer in an 80-year-old man is unlikely to increase life expectancy. A PSA test and DRE increase the detection rate of prostate cancer over DRE alone. Therefore measurement of the serum PSA value should be performed in patients in whom the identification of cancer would clearly alter BPH management (Madersbacher et al, 2004; Kaplan et al, 2006; Abrams et al, 2009). There is significant overlap between the serum PSA values of men with BPH and men with clinically localized prostate cancer. Twenty-eight percent of men with histologically proven BPH have a serum PSA value greater than 4.0 ng/mL (McConnell et al, 1994). Serum PSA trends over time (PSA velocity), measurement of free versus complexed PSA, and PSA density may help to improve the specificity of PSA in men with BPH. Newer markers such as the PCA-3 test can also help to differentiate BPH from prostate cancer. The International Prostate Symptom Score (IPSS) is recommended as the symptom scoring instrument to be used for the baseline assessment of symptom severity in men presenting with LUTS (Kaplan et al, 2006; Abrams et al, 2009). When the IPSS system is used, symptoms can be classified as mild (0 to 7), moderate (8 to 19), or severe (20 to 35). The IPSS should also be the primary determinant of treatment response or disease progression in the follow-up period. Although other symptom score questionnaires are used, the IPSS is now the U.S. and international standard. The IPSS was developed from the AUA Symptom Index (AUASI) developed by the Measurement Committee of the AUA (Barry et al, 1992a, 1992b). Each question on the IPSS can yield 0 to 5 points, producing a total symptom score that can range from 0 to 35. This seven-question set is internally consistent (Cronbach alpha, 0.85) and reliable (test-retest correlation, 0.93). The index correlates strongly with patients’ global ratings of their urinary difficulties (r = 0.78) and is sensitive to treatment response. Johnson and associates (2009) showed that patients with a low education status are more likely to misunderstand the IPSS whether they are managed in public hospital or university practice. They tend to misrepresent their symptoms and therefore may receive inappropriate treatment. Eight percent of university hospital and almost 25% of public hospital patients under-reported their moderate symptoms as mild, and 33% of university hospital and 16% of public hospital patients over-reported their mild symptoms as moderate. When administered by a medical professional, many of the inaccuracies disappear even though the questionnaire is recommended as “self-administered.” The use of the additional bother score or quality of life question may be useful in guiding appropriate treatment. Although the IPSS correlates well with quality of life measures (Sagnier et al, 1995), there is still a need for sensitive BPH-specific quality of life instruments. Furthermore, because storage symptoms often predominate there is a need for better methods of quantifying urgency, frequency, and recording any incontinence. The standardization subcommittee of the International Continence Society has usefully categorized the range of likely symptoms into three groups: storage, voiding, and postmicturition (Abrams et al, 2003). Subdividing the IPSS into the four obstructive and three storage questions may also be useful. Other systems such as the Kings Score may be more sensitive to changes in storage symptoms. A voiding diary with frequency and volume recordings also may be helpful. Additional testing should be considered after the initial evaluation if there is a significant chance the patient’s LUTS may not be due to BPH. Patients with a normal initial evaluation and only mild symptomatology on the IPSS (scores 0 to 7), or even more moderate symptoms but minimal bother, do not need additional diagnostic evaluation and can be considered for an active surveillance program and observed (Kaplan, 2006). Men who have developed serious complications of BPH should be treated surgically in most cases. Urinary flow rate, postvoid residual (PVR) urine volume, and pressure-flow urodynamic studies are appropriate tests to consider in the evaluation of men with moderate to severe symptoms (IPSS 8 to 35). The value of pressure-flow studies is debated, especially in men who elect watchful waiting or medical therapy as their management option. Cystoscopy should not be done routinely but is optional during later evaluation if invasive treatment is strongly considered. Urinary flow rate and PVR are generally recommended tests (Madersbacher et al, 2004; Kaplan, 2006; Abrams et al, 2009), and frequency-volume chart recordings are recommended by most clinicians (Madersbacher et al, 2004; Abrams et al, 2009). Surgery is generally recommended if the patient has refractory urinary retention (failing at least one attempt of catheter removal) or any of the following conditions clearly secondary to BPH: recurrent UTI, recurrent gross hematuria (resistant to 5α-reductase inhibitor therapy), bladder stones, renal insufficiency, or large bladder diverticula (McConnell et al, 1994; Denis et al, 1998; Kaplan et al, 2006; Abrams et al 2009). If there is reason to suspect that the patient’s urinary retention may be due to detrusor hypocontractility, then urodynamic studies (e.g., filling cystometry) may be helpful. Pressure-flow urodynamic studies are not informative if the patient cannot urinate. Cystoscopy is appropriate to consider before the operative procedure to help plan the most prudent approach. The presence of infection and hematuria in patients should prompt appropriate evaluation and therapy for these conditions before treatment of BPH. Uroflowmetry is either generally recommended (by the AUA [Kaplan, 2006]), recommended for specialist investigation (Sixth International Consultation [Abrams et al, 2009]), or recommended generally and required before invasive treatment (EAU [Madersbacher et al, 2004]). Uroflowmetry involves the electronic recording of the urinary flow rate throughout the course of micturition. It is a common, noninvasive urodynamic test used in the diagnostic evaluation of patients presenting with symptoms of BOO. The results of uroflowmetry are nonspecific for causes of the symptoms. For example, an abnormally low flow rate may be caused by an obstruction (e.g., hyperplastic prostate, urethral stricture, meatal stenosis) or by detrusor hypocontractility. The AHCPR Guideline Panel reached the following conclusions regarding uroflowmetry (McConnell et al, 1994), which still apply: The Fourth International Consultation on BPH concluded that flow rate measurement represents a reproducible way to quantify the strength of the urinary stream and, when used in combination with symptom scores for a small subset of patients (20%), has a high probability of correctly characterizing whether there is BOO (Denis et al, 1998). Despite its limitations, flow rate recording has demonstrated some sensitivity in identifying BOO due to BPH. Scott and coworkers (1967) and Shoukry and associates (1975) found that PFR correlated better than symptoms with the presence or absence of obstruction as determined by pressure-flow studies. Siroky and coworkers (1979) concluded that uroflowmetry was able to separate physiologically unobstructed and obstructed patients. Gleason and colleagues (1982) found that PFR distinguished between normal men and patients with BPH, urethral stricture, or prostatitis. However, they also noted that a subgroup of patients with a decompensated detrusor muscle could not be separated from the obstructed men on the basis of PFR alone. Chancellor and colleagues (1991) found that flow rate recording cannot distinguish between BOO and impaired detrusor contractility as the cause for a low PFR. None of eight measured, noninvasive urodynamic parameters was significantly different for 31 patients with outlet obstruction than for 14 patients with impaired detrusor contractility. Abrams and associates (1977, 1979) studied the value of uroflowmetry before prostatectomy. Failure rates for surgery were found to decrease with the addition of flow rate measurement to symptom assessment in preoperative evaluation. PFR appears to predict surgical outcome in some studies. In one study reported by Jensen and coworkers (1984), 53 patients underwent prostatectomy based on clinical indication alone. All three groups according to level of PFR experienced improvements in their symptom score after surgery, but the group with a PFR less than 10 mL/sec before treatment had a better overall subjective outcome as assessed by global subjective judgment. In another study, which included men studied with flow rates before and 6 months after prostatectomy (Jensen et al, 1988a), subjective evaluation revealed an overall symptomatic improvement rate of 80% after surgery. The difference in success rates for men falling above or below the cutoff value of PFR = 10 mL/sec was not significant (P = .2). When a PFR cutoff of 15 mL/sec was used, success rates for men above or below the cutoff value differed significantly. McLoughlin and coworkers (1990), using urodynamic testing and a cutoff value of 12 mL/sec, evaluated 108 men with clinical BPH before and 1 year after surgery and determined that if using a PFR less than 12 mL/sec as an indicator for obstruction then only 3% of patients would have been subjected to an unnecessary TURP. These authors believed that the routine pressure-flow studies or cystometrograms were not indicated but that the screening of flow rates followed by further urodynamic testing in patients with a PFR of greater than 12 mL/sec should be considered. Very low rates do not appear to portend poor treatment outcome. In one study of 84 patients undergoing surgery for symptomatic BPH (Donkervoort et al, 1975), patients with a preoperative PFR less than 7 mL/sec improved symptomatically as much as patients with a PFR greater than 7 mL/sec. Postvoid residual urine volume is the amount of fluid remaining in the bladder immediately after the completion of micturition. Studies indicate that PVR urine volume normally ranges from 0.09 to 2.24 mL, with the mean being 0.53 mL (Hinman and Cox, 1967). Seventy-eight percent of normal men have PVR volumes of less than 5 mL, and 100% have volumes of less than 12 mL (DiMare et al, 1963). The Agency for Health Care and Policy Research BPH Guideline Panel reached the following conclusions regarding PVR urine volume (McConnell et al, 1994): The Fourth International Consultation initially recommended PVR volume determination in the initial assessment and during monitoring of patients under watchful waiting or other conservative treatment regimens (Denis et al, 1998). PVR urine volume measurement can be performed by noninvasive (ultrasound) and invasive (catheterization) methods. Invasive techniques are accurate if performed correctly but carry a small, but clinically significant, risk of discomfort, urethral injury, UTI, and transient bacteremia. Small portable and less expensive devices can be used to measure the PVR urine volume with reported accuracy and are comparable to more expensive ultrasound units and catheterization. Birch and coworkers (1988) reported that of 30 men with BPH, 66% had wide variations in PVR urine volume when three measurements were done on the same day. In 34% of patients there was no difference among the three measurements. In 58%, at least two volumes were significantly different. In 8% of patients, all three were different. In most patients, two measurements were statistically similar whereas the third one yielded quite different results. Bruskewitz and colleagues (1982) found similarly wide variations of the measured amount when they performed repetitive measurements of PVR urine volume (repeated two to five times) by in-and-out catheterization on 47 men before prostatectomy. They also found no correlation between the amount of residual urine and any cystoscopic or urodynamic findings, symptoms, or the presence or absence of a history of UTIs. Most clinical studies demonstrate minimal correlation between PVR urine volume and baseline measurements of symptoms, flow rate, or urodynamic measures of obstruction (Griffiths and Castro, 1970; Shoukry et al, 1975; Abrams and Griffiths, 1979). However, Neal and associates (1987) found a significant association in 253 men between PVR urine volume, age, “below normal” PFR, and high urethral resistance. Low voiding pressure, however, did not correlate well with the PVR amount. The authors concluded that outflow obstruction is related to the development of increasing amounts of PVR urine. In the AUA Outcome Study, Barry and colleagues (1993) found a significant correlation between high PVR urine volumes and low flow rates but no correlation with IPSS. Traditionally, urologists have assumed that increasing amounts of PVR urine denote BPH progression and are thus an “indication” for surgery. This concept underlies the common inclusion of PVR urine volume in each individual government’s appropriateness criteria. Unfortunately, data are lacking to support the predictive value of PVR urine volume. Andersen (1982) studied 104 men with BPH and reported two patterns of BPH progression. The slow course was characterized by the development of high levels of PVR urine that resulted in decompensation of the detrusor muscle and eventually led to urinary retention. The fast course was associated with uninhibited detrusor contractions. The amount of PVR urine, the presence of uninhibited detrusor contractions, and symptoms correlated poorly in the study. Nevertheless, Andersen recommended the PVR urine volume as a safety parameter when measured longitudinally throughout the clinical course of a patient with prostatism. If the initial evaluation, flow rate, and PVR urine volume are not sufficiently suggestive of BOO, further urodynamic assessment by pressure-flow studies should be considered, especially if an invasive treatment is considered (i.e., surgery) or if surgical treatment has failed (McConnell et al, 1994; Denis et al, 1998; Kaplan et al, 2006; Abrams et al, 2009). Pressure-flow studies differentiate between patients with a low PFR secondary to obstruction and those whose low PFR is caused by impaired detrusor contractility. These studies should be performed when the distinction between the two will affect therapeutic decisions. Patients with a history of neurologic diseases known to affect bladder or sphincteric functions, as well as patients with normal flow rates (PFR > 15 mL/sec) but bothersome symptoms, may also benefit from urodynamic evaluation and especially if surgical therapy is contemplated. The value of pressure-flow measurement in predicting treatment outcome is uncertain. In a study by Abrams and colleagues (1979), the inclusion of pressure-flow data in the preoperative evaluation and indication for surgery reduced the subjective failure rate to 12%, down from 28% when patients were certified as candidates for surgery without the urodynamic data. However, a 28% failure rate is significantly higher than that reported in other TURP series (McConnell et al, 1994). Jensen and Andersen (1990) recommended invasive urodynamic testing for patients with a PFR greater than 15 mL/sec. For the population in their study this would have resulted in an additional 9% of patients being excluded from surgery and a decrease in failure rate to 8.3%. The support for this recommendation has to be questioned, however, in light of an earlier study by Jensen and coworkers (1988b, 1988c) that found most unsatisfied patients are incorrectly classified preoperatively even with urodynamic testing. Pressure-flow studies do permit more accurate categorization of patients. Abrams and associates (1979) used pressure-flow plots in addition to flow rate measurement. The study found that in about half the cases the patients with LUTS could be correctly classified as obstructed or nonobstructed by PFR alone but that the addition of the detrusor pressure (Pdet) at PFR allowed correct classification in two thirds of the group. The remaining one third of the patients were assessed by pressure-flow plot. In many of these patients, both Pdet and PFR were low, indicating a decompensating detrusor muscle as the source for the low PFR. Urethrocystoscopy is not recommended to determine the need for treatment. Although the linkage between the endoscopic appearance of the lower urinary tract and the treatment outcome is poorly documented, available information suggests that the relationship is minimal (McConnell et al, 1994). The test is recommended for men with LUTS who have a history of microscopic or gross hematuria, urethral stricture disease (or risk factors such as history of urethritis or urethral injury), bladder cancer or suspicion of carcinoma in-situ, or prior lower urinary tract surgery (especially prior TURP). Urethrocystoscopy may be considered in men with moderate to severe symptoms who have chosen (or require) surgical or other invasive therapy to help the surgeon determine the most appropriate technical approach. Upper urinary tract imaging is not recommended in the routine evaluation of men with LUTS unless they also have one or more of the following: hematuria, UTI, renal insufficiency (ultrasonography recommended), history of urolithiasis, or history of urinary tract surgery (McConnell et al, 1994; Denis et al, 1998; Kaplan et al, 2006; Abrams et al, 2009). Intravenous urography before BPH treatment was performed by 73.4% of urologists in the United States in the late 1980s (Holtgrewe et al, 1989) and is associated with a 0.1% incidence of significant adverse events. Generally, ultrasound imaging is now preferred but is unnecessary in the uncomplicated case. The presence or history of hematuria, renal insufficiency, UTI, and/or history of stones or prior urinary tract surgery increases the likelihood that imaging will demonstrate clinically significant findings (Juul et al, 1989; Andrews et al, 2002). Although there are no conclusive data on the combined incidence of the important clinical predictors just listed, approximately one third of all men with BPH have one or another indication for urinary tract imaging. The clinical consequences of BPH includes LUTS and associated reduction of quality of life, detrusor dysfunction characterized by detrusor acontractility, detrusor instability, and detrusor fibrosis; incomplete bladder emptying; acute and chronic urinary retention; UTI; renal insufficiency; and hematuria (Shapiro and Lepor, 1995). The goals of treatment for BPH include relieving LUTS, decreasing BOO, improving bladder emptying, ameliorating detrusor instability, reversing renal insufficiency, and preventing disease progression, which may include a deterioration of symptoms, future episodes of gross hematuria, UTI, AUR, or the need for surgical intervention. The primary objective of the AUASI (now IPSS) was to provide a universally accepted instrument to quantify the impact of BPH therapies on LUTS (Barry et al, 1992a, 1992b). There is no standardized format for reporting changes in the AUASI or other quantitative indices of symptom severity after treatment. Symptom response has been reported as a percentage of patients achieving a threshold response or as group mean changes in a symptom score. The literature typically reports the percentage of men achieving between a 30% and a 50% reduction in the symptom score. Expressing the symptom response as a single threshold response does not discriminate the overall magnitude of the clinical effect. When the baseline symptom scores are mild to moderate, small and clinically insignificant changes correspond to large percentage changes. When baseline symptom scores are severe, relatively large absolute changes may not be clinically significant. Symptom outcome should be expressed both as a percentage of patients achieving a threshold reduction response and as group mean changes in the symptom score. The clinical significance of changes in the AUASI score were reported by Barry and colleagues (1995). There were 1165 subjects who participated in a randomized double-blind placebo-controlled study of medical therapy and completed the AUASI at baseline and after 3 months of treatment. The absolute and percentage changes in AUASI and BPH Impact Index scores were correlated with five global ratings of symptom improvement. The group mean changes in AUASI for subjects rating their improvement as markedly, moderately, or slightly improved, unchanged, or worse were −8.8, −5.1, −3.0, −0.7, and +2.7, respectively. The relationship between the patients’ global ratings of improvement and the AUASI and BPH Impact Index changes were dependent on the baseline AUASI score. This important study provides the data required to determine sample sizes and interpret the clinical significance of symptom improvement in BPH clinical trials. A 3-point change appears perceptible to symptomatic men. Experimental animal models of BOO have demonstrated profound changes in bladder ultrastructure, cellular composition, metabolism, and function resulting from BOO (Levin et al, 2000). These experimental observations must be cautiously extrapolated to man, because the response to BOO depends on the species and the severity and duration of obstruction. Animal studies demonstrate that under experimental conditions BOO causes alterations likely to adversely affect bladder function. The justification for measuring and treating BOO in men with BPH is to reverse or prevent these deleterious consequences of BOO. Uroflowmetry represents a noninvasive and inexpensive but indirect indicator of urinary performances measure of BOO (Siroky, 1990). The reporting of PFR has been standardized (Abrams et al, 2003). At the lower spectrum of PFR a relatively small absolute change (i.e., 4 to 6 mL/sec) corresponds to a relatively high percentage change, whereas at the higher end of PFR a relatively large absolute change (i.e., 12 to 17 mL/sec) corresponds to a relatively modest percent change. The clinical significance of the changes in PFR cannot be defined, owing to the lack of correlations with relevant clinical, physiologic, or biochemical outcomes. The clinical significance of PVR urine volume measurement is controversial. Barry and colleagues (1993) reported no correlation between AUASI score and PVR amount. It has been suggested that the PVR urine volume may predispose to UTI and irreversible bladder dysfunction secondary to stasis and overdistention. There are no data clearly documenting that the incidence of UTI is related to the PVR urine volume. Another limitation of PVR urine measurements is variability over short intervals of time (Bruskewitz et al, 1982). It is imperative to measure the PVR volume on several occasions if this parameter will influence treatment decisions. The definition of bladder overactivity (detrusor instability) is the development of a detrusor contraction exceeding 15 cm H2O at a bladder volume less than 300 mL (Jepsen and Bruskewitz, 2000). The clinical significance of an overactive bladder (OAB) in men with BPH is unresolved. There is no evidence that men with detrusor instability electing watchful waiting are predisposed to develop disease progression. The presence of an OAB does not reliably predict response to medical or surgical treatment. Therefore improvement of an OAB is not a standard outcome measure in clinical trials. A significant proportion of men with LUTS will not choose medical or surgical intervention because the symptoms are not bothersome, the complications of treatment are perceived to be greater than the inconvenience of the symptoms, and there is a reluctance to take a daily pill owing to side effects and/or the cost of treatment. Reassured that the symptoms are not caused by cancer or other serious genitourinary pathology, or that the delay in treatment will not have irreversible consequences, watchful waiting is often the patient-driven treatment of choice in the absence of absolute indications for intervention. Of 670 consecutive men with BPH referred to 39 urologists in the Netherlands, 41% elected watchful waiting (Stoevelaar et al, 1999). It is unreasonable to discourage an informed patient with severe symptoms and no other consequences of BPH from pursuing watchful waiting despite the safety and effectiveness of medical therapy. Watchful waiting does not imply the total absence of intervention. The severity and bother due to symptoms may be improved by simple measures such as decreasing total fluid intake especially before bedtime, moderating the intake of alcohol- and caffeine-containing products, and maintaining timed voiding schedules. The impact of watchful waiting was examined in a study of 556 subjects with moderate symptoms of BPH randomized to TURP versus watchful waiting (Wasson et al, 1995). The changes in all outcome measures were significantly greater in the TURP group. A relevant outcome for patients selecting watchful waiting is disease progression. During 3 years of follow-up, treatment failure was observed in 23 (8.2%) and 47 (17%) of subjects randomized to TURP and watchful waiting, respectively. Treatment failure in the watchful waiting group was most often the result of increasing PVR volume or symptom score. Significant renal impairment was not seen. Brown and associates (2007) evaluated the effectiveness of self-management as a first-line intervention for men with LUTS in a randomized controlled trial set in a teaching hospital and a district general hospital in London. One hundred forty men were randomized between standard care and “self-management,” which comprised three small group sessions of relevant urinary education and lifestyle advice. Self-management significantly reduced the frequency of treatment failure and reduced urinary symptoms. Because of the large observed benefit of self-management, these investigators suggested a large multicenter trial to confirm whether self-management could be considered as first-line treatment for men with LUTS. Prior to the 1980s, prostatectomy was the only widely accepted intervention for BPH. The enthusiasm for medical therapy has been supported in part by the limitations of prostatectomy, which include the morbidity of the surgical procedure, failure to invariably achieve a successful outcome, and a small but significant re-treatment rate (Lepor, 1993). Although medical therapies do not achieve the same level of efficacy as prostatectomy, the attractive features of medical therapy relative to prostatectomy are that clinically significant outcomes are obtained with fewer, less serious, and reversible side effects (Lepor, 1993). Because the indication for intervention in the overwhelming majority of patients with BPH is to improve quality of life by relieving symptoms (Emberton et al, 2008), the lower morbidity of medical therapy is of paramount importance in patient-driven treatment decisions. In 1990, TURP was second only to cataract surgery in terms of expenditures paid by the U.S. Medicare program. Medical therapy is currently considered the preferred treatment alternative for those individuals who lack absolute indications for surgery. Because the overwhelming majority of men undergoing TURP lack absolute indications for intervention (Mebust et al, 1989) and prefer nonsurgical options (Emberton et al, 2008), the number of prostatectomies performed throughout the world has decreased significantly. A survey of the U.S. Medicare database also revealed that the absolute number of prostatectomies decreased from 250,000 in 1987 to 116,000 in 1996 to 88,000 in 2000 (Wasson, 2000) and stabilized since then. This 55% reduction in TURP has occurred despite the progressively increasing number of men enrolled in the Medicare program and overall increase in U.S. spending on BPH (Wei et al, 2005). Similar reductions in TURPs have been reported from France, Canada, Denmark, and Germany. Approximately 30% of American men older than 50 years have moderate to severe symptoms (Chute et al, 1993; Lepor and Machi, 1993). Based on the demographics of the U.S. population, 6.5 to 8.7 million men are eligible to discuss BPH treatment options (Wei et al, 2005; Jacobsen et al, 1995). The overwhelming majority of these men would not elect prostatectomy, owing to the risks associated with surgical intervention. These individuals are potential candidates for medical therapy. A potential role of medical therapy is to prevent the development of BPH or its progression. There are several factors limiting the enthusiasm for preventing the development of BPH. The clinical manifestations of BPH are rarely life threatening. Preventative intervention would have to be initiated before the fifth decade of life coinciding with the development of BPH (Partin, 2000). The long-term exposure to drug-induced adverse events and the prohibitive costs are the primary limitations of prevention therapy. In addition, effective medical and surgical therapy exists when BPH ultimately does becomes clinically evident. Because there are no clinical, biochemical, or genetic predictors of BPH development or progression, every man is potentially at risk. The ability to identify those individuals who are predisposed to develop clinical BPH refractory to medical therapy would provide a more compelling rationale for prophylaxis. There is good evidence that men with very large prostates (and usually higher PSA values) are at greater risk for developing urinary retention (Jacobsen et al, 1997) and that medical therapy (finasteride or dutasteride) can significantly decrease this risk of developing urinary retention (McConnell et al, 1998; Roehrborn et al, 2004). The decision to offer preventative therapy for urinary retention depends on the risk of the events, cost associated with treatment, and patient preferences for intervention. The rationale for α-adrenergic blockers in the treatment of BPH is based on the hypothesis that the pathophysiology of clinical BPH is in part caused by BOO, which is mediated by α1-adrenergic receptors associated with prostatic smooth muscle (Caine, 1986) (Fig. 92–4). The importance of this dynamic obstruction was supported by morphometric studies demonstrating that smooth muscle is one of the dominant cellular constituents of BPH, accounting for 40% of the area density of the hyperplastic prostate (Shapiro et al, 1992). Caine and coworkers (1975) reported that the human prostate contracts in the presence of the α-adrenergic agonist norepinephrine. Several investigators subsequently demonstrated that the tension of prostate smooth muscle is mediated by the α1 receptor (Hieble et al, 1985; Lepor et al, 1988; Gup et al, 1989). Lepor and colleagues (1988) were the first investigators to characterize the α1 receptor in the human prostate using radioligand binding studies. These investigators subsequently reported that 98% of the α1 receptors are localized to the prostatic stroma (Kobayashi et al, 1994). The importance of the adrenergic innervation of the prostate was further supported by the observation of high levels of norepinephrine in the human prostate (Lepor et al, 1990). Although the finding of high levels of smooth muscle α1 receptors and norepinephrine in the human prostate suggests an important role of the adrenergic innervation in prostatic function, it cannot be assumed that these factors are directly responsible for clinical BPH. Lepor and associates (1990) reported no significant differences between norepinephrine levels, α1 receptor density, or isometric contractile responses to phenylephrine (Gup et al, 1989) in BPH tissues obtained from men with symptomatic and asymptomatic BPH. Other investigators have shown α1 receptor levels are higher in prostatic adenoma relative to prostatic capsule (Yamada et al, 1987; Kawabe et al, 1990). These observations simply show regional differences of α1 receptors in the prostate and do not prove that clinical BPH is caused by upregulation of the α1 receptor. The most definitive evidence that blockade of prostate α1 receptors relieves BOO was the observed direct relationship between the area density of prostate smooth muscle and the change in the PFR in 26 subjects undergoing prostatic biopsy before initiating α-adrenergic blocker therapy with terazosin (Hytrin) (Shapiro et al, 1992). Although the prostates of those subjects achieving symptom improvement had a significantly greater group mean area density of smooth muscle compared with those of nonresponders, a direct relationship between prostate smooth muscle area density and change in symptom scores was not observed. These observations suggest that nonprostate smooth muscle–mediated α1 receptor events may also be responsible for the effectiveness of α-adrenergic blockade and that α1 receptor–mediated symptom improvement and decreases in BOO are mediated by different mechanisms. α-Adrenergic blockers may be classified according to α receptor selectivity and serum elimination half-life (Table 92–1) Table 92–1 Classification of α-Adrenergic Blockers and Recommended Doses Phenoxybenzamine, a nonselective α blocker, was shown to be highly effective for BPH (Caine et al, 1976, 1978). The limitation of phenoxybenzamine was the high incidence and severity of adverse clinical events. Berthelsen and Pettinger (1977) described two subtypes of the α receptor (α1 and α2). Prazosin was one of the first α1 receptor antagonists to be investigated for the treatment of BPH (Hedlund et al, 1983). The efficacy of phenoxybenzamine and prazosin are comparable; however, prazosin is better tolerated, implying that efficacy and toxicity are mediated primarily by the α1 and α2 receptors, respectively (Lepor, 1989). Prazosin and other α1 antagonists, including intermediate-release (IR) alfuzosin (Jardin et al, 1991) and indoramin (Ramsay et al, 1985), require at least twice-daily dosing, owing to the relatively short serum elimination half-lives. The next advance in the development of α-adrenergic blockers was the development of advanced drugs with serum elimination half-lives that allowed for once-a-day dosing. Terazosin (Hytrin) (Lepor et al, 1992) and doxazosin (Cardura) (Gillenwater et al, 1995), tamsulosin (Flomax) (Chapple et al, 1997; Narayan and Tewari, 1998; Wilt et al, 2002b), and extended-release alfuzosin (UroXatral) (McNeill et al, 2005; van Kerrebroeck et al, 2000) are long-acting α-adrenergic blockers that have been shown to be safe and effective for the treatment of BPH. Molecular cloning studies have identified three subtypes of the α1 receptor (Andersson et al, 1997). Price and coworkers (1993) reported that the mRNA encoding the α1A receptor is predominant in the human prostate. The fact that the α1A mRNA is translated does not mean the encoded protein is translated. Lepor and associates reported that using autoradiographic (Kobayashi et al, 1994) and immunohistochemical (Walden et al, 1997) techniques, the α1A and α1B receptors are predominant in the human stroma and epithelium, respectively. Prostate smooth muscle tension has been shown to be mediated by the α1A receptor (Forray et al, 1994). Tamsulosin is a once-daily administered α1 antagonist that exhibits some modest degree of selectivity for the α1A versus the α1B receptor and no selectivity for the α1A versus the α1D receptor (Foglar et al, 1995). The pharmaceutical industry has developed α1 antagonists that are 1000-fold selective for the α1A receptor versus α1B/α1D (Forray et al, 1994). Recently, silodosin (Rapaflo) has been introduced. This agent shows 162:1 selectivity for α1A versus α1B adrenoceptors and is achieving promising results. Multicenter, randomized, placebo-controlled studies have consistently shown that symptom and flow improvement is dependent on the dose of the α1 blockers. The differences between the effectiveness of different doses were often not statistically significant because these dose-ranging studies were not adequately powered to show significant differences between dose groups. MacDiarmid and coworkers (1999) have provided the most compelling evidence for a positive correlation relationship between dose and effectiveness of α1 blockers in the treatment of BPH. Responders to 4 mg of doxazosin were randomized in a double-blind manner to receive 4 mg or 8 mg of doxazosin. The improvement observed in the 8-mg group was 3.7 symptom units greater than in the 4-mg group (P = .03). In phase 3 trials, the impact of dose observed in the responders is diluted by the lack of effect in the nonresponders. In clinical practice, nonresponders are withdrawn from treatment. Several reviews have summarized the extensive clinical experiences with α-adrenergic blockade in BPH (Chapple, 1998; Djavan and Marberger, 1999; Lowe, 1999; Lepor, 2000; Roehrborn et al, 2004; Kaplan, 2008). Nonselective and short-acting α1 antagonists are used less commonly in clinical practice, owing to tolerance and the requirement for multiple daily dose. Randomized, double-blind, placebo-controlled studies have reported the safety and efficacy of phenoxybenzamine (Caine et al, 1978; Abrams et al, 1982), prazosin (Hedlund et al, 1983; Kirby et al, 1987; LeDuc et al, 1990; Ruutu et al, 1991; Chapple et al, 1992), indoramin (Iacovou and Dunn, 1987; Chow et al, 1990; Stott and Abrams, 1991), and IR alfuzosin (Ramsay et al, 1985; Carbin et al, 1991; Jardin et al, 1991; Hansen et al, 1994). With the exception of alfuzosin, these studies typically enrolled relatively small numbers of subjects into short-term single-dose studies without quantitative assessment of symptom improvement. Multicenter, randomized, double-blind, placebo-controlled studies have examined the safety and efficacy of the long-acting α-adrenergic blockers terazosin, doxazosin, tamsulosin, and slow-release (SR) alfuzosin. Subjects enrolled in these studies generally presented with moderate to severe symptoms, PVR less than 300 mL, and no absolute indications for surgical intervention. Representative studies are reviewed to illustrate the safety, efficacy, and most effective use of α-adrenergic blockers in BPH. The reader is referred to the original articles for more comprehensive outcome assessments. Randomized, double-blind, multicenter, placebo-controlled studies have consistently demonstrated the efficacy and safety of terazosin for BPH (Di Silverio, 1992; Lepor et al, 1992; Lloyd et al, 1992; Brawer et al, 1993; Elhilali et al, 1996
Diagnosis
Initial Evaluation
Physical Examination
Urinalysis
Serum Creatinine Measurement
Serum Prostate-Specific Antigen
Symptom Assessment
Additional Diagnostic Tests
Diagnostic Tests in Men Who Require Surgery for Benign Prostatic Hyperplasia
Uroflowmetry
Postvoid Residual Urine Volume
Pressure-Flow Studies
Urethrocystoscopy
Imaging of the Upper Urinary Tract
Assessing the Effectiveness and Safety of Medical Therapy
Clinical End Points
Quantitative Outcome Measures
Symptoms
Bladder Outlet Obstruction
Bladder Emptying
Bladder Overactivity
Nonsurgical Therapy
Watchful Waiting or “Self-Help”
Medical Therapy
The Impact of Medical Therapy
Preventing Benign Prostatic Hyperplasia with Medical Therapy
Therapy with α-Adrenergic Blockers
Rationale
Classification of α-Adrenergic Blockers
CLASS OF α-ADRENERGIC BLOCKER
DOSE
Nonselective
Phenoxybenzamine
10 mg bid
α1
Prazosin
2 mg bid
IR Alfuzosin
2.5 mg tid
Indoramin
20 mg bid
Long-Acting α1
Terazosin
5 or 10 mg qd
Doxazosin
4 or 8 mg qd
Alfuzosin SR
10 mg qd
Subtype Selective
Tamsulosin
0.4 mg qd
Silodosin
8 mg qd
Interpreting the α-Adrenergic Blocker Literature
Dose Response
Review of the Literature
Terazosin
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree