2.
The differential diagnosis of allograft AKI changes with time following transplant, whereas the differential diagnosis of native AKI (prerenal, renal, and postrenal) can often be considered without regard to the chronology of a specific event.
3.
The level of proteinuria that is associated with future graft dysfunction has been incompletely defined, unlike specific causes of native kidney diseases.
4.
AKI in the allograft recipient often is an asymptomatic outpatient presentation, unlike native AKI which is typically an inpatient or emergency room presentation.
With these differences in mind and in the absence of a workgroup-defined definition, one must rely upon general consensus regarding the degree to which transplant graft dysfunction is clinically significant and requires further investigation. A reasonable working definition of transplant graft dysfunction is:
1.
A rise in serum creatinine of 20 % over an established baseline, or
2.
A failure of the serum creatinine to fall following transplant surgery, or
3.
Proteinuria >300 mg per day (with caveats regarding the timing of measurement of proteinuria post-transplant, discussed below)
The Differential Diagnosis of Transplant Graft Dysfunction
A logical approach to the patients with allograft dysfunction can begin by considering transplant-related causes of prerenal, renal, and postrenal injury (Table 14.1).
Prerenal: The most common cause of prerenal azotemia in kidney transplantation is due to calcineurin inhibitor (CNI) use. This includes the medications cyclosporine (Neoral, Gengraf) and tacrolimus (Prograf). While the chronic effect of CNIs upon graft function is a subject of research and debate, it is well accepted that CNIs can cause AKI by vasoconstrictive effects on the afferent arteriole. These changes can occur within what is considered therapeutic trough ranges of these agents (e.g., a change in tacrolimus trough from 5 to 9 ng/ml). Other transplant-specific causes of AKI include complications of the surgical procedure such as arterial or venous thrombosis and anastamotic stricture (e.g., transplant renal artery stenosis). General causes of prerenal azotemia are quite common, including hypoperfusion related to hypovolemia and/or hypotension. Often small changes in volume status, renal blood flow, or blood pressure can result in AKI while patients are exposed to CNIs.
Intrarenal: Consideration of the potential location of renal injury can be helpful in formulating a differential diagnosis of graft dysfunction.
Tubulointerstitial: AKI related to ischemia reperfusion injury leading to acute tubular necrosis is related to donor factors such as age, cold ischemia time, and degree of warm ischemia time in the setting of donors who undergo organ procurement following circulatory arrest (see Chaps. 9 and 13, “Delayed Graft Function”). Acute cellular rejection remains a critical consideration in the assessment of transplant AKI. Tubulointerstitial inflammation in this setting is a predominantly T-lymphocyte-mediated process that has become less common over previous decades with the introduction of more potent immunosuppressive agents [3] (Table 14.2). However, depending upon risk factors of the donor and recipient and the immunosuppressive agents used, acute rejection rates may range from 10 % for the lowest risk patients to 40 % for the highest risk patients within the first year. With potent immunosuppression comes an increased risk of infection, another common intrarenal cause of graft AKI. The allograft is sensitive to ascending bacterial infections due to the lack of a natural ureterovesicular valve to prevent reflux, and transplant pyelonephritis can present rapidly after the early signs of cystitis, causing tubulointerstitial injury. Viral infections (commonly BKV, less commonly cytomegalovirus or adenovirus) also can cause AKI due to tubulointerstitial injury. Rarely, post-transplant lymphoproliferative disorder may present with graft dysfunction due to extensive lymphocyte infiltration of the allograft (see Chap. 25).
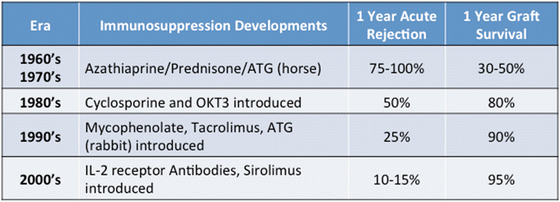
Table 14.2
Timeline of immunosuppression regimens and allograft outcomes

Vascular: More severe forms of acute rejection that are reflected by monocyte infiltration to the intimal layers of the arterioles (Banff grade II) or neutrophil infiltration of the peritubular capillaries (suggestive of antibody-mediated rejection, see Chap. 29) can occur and often are associated with under-immunosuppression. Severe forms can present as a thrombotic microangiopathy (TMA). Drug-associated TMA must also be considered, as there appears to be an association of TMA with CNI, with some reports of a higher propensity of TMA when CNIs are used in conjunction with MTOR inhibitors (sirolimus, everolimus) [4].
Glomerular: The finding of proteinuria with or without a change in GFR requires consideration of the patient’s primary disease that led to renal failure, as a number of glomerulopathies may recur following transplant (see Chap. 15). The possibility of the development of a de novo glomerulonephritis must also be considered. Additionally, the potential development of transplant glomerulopathy, often associated with indolent antibody-mediated injury causing glomerular basement membrane duplication, is common following transplant and associated with a particularly poor graft prognosis [5].
Postrenal: Specific transplant-related causes of postrenal graft dysfunction include ureteral stricture, which may be due to anastamotic complications or to ureteral ischemia. While not definitive, an association of BKV infection/reactivation and ureteral stricture has been reported [6]. The ureter may become obstructed by compression of a fluid collection in the weeks following surgery, including hematoma, seroma, or lymphocoele. Finally, injury to the ureter can lead to urinary leak and urinoma formation, which may also compress the ureter leading to allograft dysfunction. This latter complication often presents clinically with pain at the site of urine leak, as urine can cause severe irritation of the peritoneal membrane.
Beyond these transplant-related causes of graft dysfunction, the differential diagnosis of AKI for native kidney disease should also be considered, including drug toxicities, IV contrast exposure, bladder dysfunction or prostatic hypertrophy, and systemic diseases that may influence renal blood flow.
The Influence of Time Post-transplant in the Differential Diagnosis of Allograft Dysfunction
A comprehensive assessment of the possible causes of allograft dysfunction is important since many of these etiologies can present at any time following transplant. However, the frequency of most of these causes follows a pattern that can aid the clinician in focusing upon likely causes depending on whether the assessment is immediately (within 1 week) following transplant, early (1–12 weeks) following transplant, or late (after 3 months) from transplant (Table 14.1).
Immediate Causes of Graft Dysfunction (Within 1 Week Following Surgery)
During the first week following transplant, prerenal causes predominate, due to ischemia–reperfusion injury-related acute tubular necrosis, hemodynamic effects, and rare circumstances of vascular complications. Hematoma formation may cause ureteral compression as early as 1 week following surgery. Heavy proteinuria due to recurrent focal glomerulosclerosis can lead to hemodynamic effects at this time. Of note, it may be difficult to distinguish proteinuria from native kidney versus transplant source in the setting of the patient transplanted prior to the need for dialysis or with residual renal function. In general, proteinuria from native kidneys tends to dissipate over 4–12 weeks after surgery presumed due to alterations in renal perfusion [7]. However, severe nephrotic range proteinuria even early following transplant requires consideration of transplant graft etiology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






