(1)
Division of Thoracic Surgery, Department of Surgery, Weill Cornell Medical College, Houston Methodist Hospital, 6565 Fannin Street, Houston, TX 77030, USA
(2)
Division of General Thoracic Surgery, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA
Keywords
EndoscopyEndoscopeEndoscopic ultrasoundBiopsyEndoscopic mucosal resectionEsophageal ablationMucosectomySubmucosal dissectionBarrett’sDysplasiaSquamous cell liningEsophageal cancerAdenocarcinomaSquamous cell cancerHigh definition endoscopyThe art of surgical endoscopy has revolutionized the diagnosis and treatment of gastrointestinal disorders. Gastroenterologists and esophageal surgeons must familiarize themselves with the proper technique of examining a patient for upper gastrointestinal endoscopy.
Current endoscopic instrumentation includes a modern video high-definition endoscope, illustrated in Fig. 3.1. The video camera is connected to a control panel that can capture images, adjust lighting, and change views. At the terminal end of the insertion tube are an air or water nozzle, an objective lens, a biopsy and suction channel, and an illuminating lens. The biopsy channel allows the passage of biopsy forceps or instrumentation for cytologic testing as well as therapeutic maneuvers. The flexible end of the tube allows for atraumatic insertion, manipulation, and guidance within the esophageal lumen. A deflection control knob with a locking switch makes it possible to look up and down and right and left. Other parts of the system include an instrument port; suction, air, and water valves; suction, video, and water container connectors; and a universal cord that connects the handheld instrument to the processor.
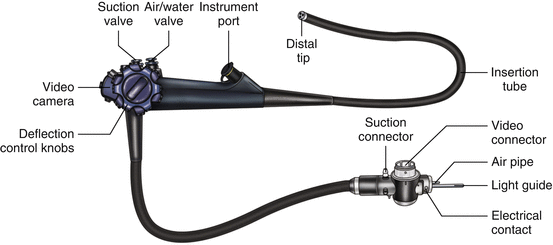

Fig. 3.1
The parts of a standard Olympus endoscope (Olympus America, Center Valley, PA)
The newer versions of endoscopes are equipped with a tiny camera at the distal end of the instrument, which transmits the digitalized image to an external television monitor for viewing. Typically, most are high definition. Carbon dioxide can be insufflated to minimize air entrapment. Developments in this technology have added special lenses to the tip of the instrument for magnification, and diagnostic ultrasound has been adapted to the instrument to facilitate the elucidation of cardiac and gastrointestinal disorders.
Esophagogastroduodenoscopy (EGD)
Indications
There are several indications for EGD examination of the upper gastrointestinal (GI) tract:
Investigations of symptoms such as dysphagia, odynophagia, persistent pyrosis, hematemesis, or melena
Retrieval of a foreign body
Evaluation of unremitting upper abdominal pain with a normal upper GI barium examination, or suspicious abnormalities identified on a barium upper GI series
Endoscopy Procedure
A patient being considered for any esophageal surgery should undergo endoscopic examination prior to their operation, to ensure that there have been no pathologic changes since the last endoscopy and to ensure that the tumor or abnormality is as described when the patient was initially referred. Additionally, a patient with a previous surgery should undergo surveillance endoscopy to evaluate the anastomosis for recurrence and the pylorus for patency; this endoscopy ideally should be performed by the surgeon who best understands the patient’s reconstructed anatomy.
Preparing for the Endoscopy
The patient should not be fed for 6–8 h prior to an upper endoscopy. Antireflux medication may be administered prior to the exam. In an emergency, rapid-sequence intubation may protect the airway of a patient who has recently consumed a meal.
Technique
The posterior pharynx is anesthetized with topical anesthetic spray or gargle, unless the procedure is being performed under general anesthesia.
Intravenous sedation is administered slowly until the patient is comfortable. For more complicated endoscopic procedures, we advocate the use of general anesthesia and intubation to avoid aspiration.
A patient who is intubated may be left in the supine position. Otherwise, with the patient in the left lateral decubitus position, the endoscope may be passed in one of two ways:
A mouth guard is positioned between the patient’s teeth, and the scope is advanced under direct vision (Fig. 3.2). The epiglottis and vocal cords are visualized and the instrument is passed posteriorly into the esophageal orifice. This technique allows visualization of the hypopharynx and may be particularly useful in patients with suspected lesions high in the esophagus or pharynx, or in relatively uncooperative patients.
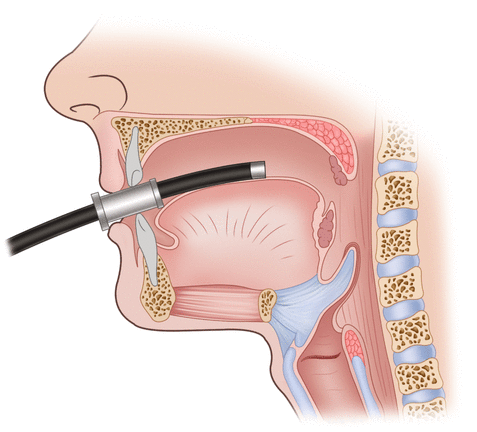
Fig. 3.2
A mouthguard is typically used for insertion of the endoscope, to avoid damage to the patient’s dentition and the endoscope
In an alternative method, the instrument is passed by feel of the operator. The endoscopist does not attempt to observe the lumen until the scope is in the esophagus. Observation of the upper pharynx and vocal cord are performed after removal of the scope. The index and middle fingers of the endoscopist are placed over the tongue to the posterior pharynx; the scope is then placed on top of the fingers and manipulated downward to the esophageal orifice. The guiding fingers ensure that the scope glides along the wall of the pharynx and is centered.
As the patient swallows, the instrument is advanced into the esophagus. Undue force is never applied and the scope is advanced only when the lumen can be visualized, to avoid perforation of the pharynx. (Perforation of the pharynx most likely would involve the piriform sinus, and the result would be massive injection of subcutaneous air throughout the neck and mediastinum when the endoscope is insufflating the hypopharynx.)
The esophagus is inflated with air and inspection is begun. The normal esophagus demonstrates a long, round, tubular lumen with a pale, smooth mucosa.
Careful inspection of the mucosal surfaces is important to detect subtle irregularities that may herald underlying pathology. When an irregularity of the mucosa is encountered, a biopsy specimen and brushings can be obtained for cytologic examination, after ensuring (using the Doppler flow tool) that the abnormality is not a vascular structure. If there is concern about the structure and depth, then an ultrasound may be used to determine the characteristics of the lesion.
At the most distal end of the normal esophagus, about 40 cm from the incisors, there is a sharp transition from the pale esophageal (squamous) mucosa to the deeper orange gastric (columnar) mucosa (Fig. 3.3a, b). This junction is called the Z-line and is usually somewhat irregular. An abnormal mucosal tongue of columnar mucosa is representative of Barrett’s island, and biopsies must be performed to confirm metaplasia (Fig. 3.3c). Large extensions of gastric-type mucosa above this point may represent Barrett’s esophagus (columnar metaplasia of the esophagus). A biopsy specimen should be obtained from such an area, as dysplasia and malignancy may arise within a Barrett’s epithelium.
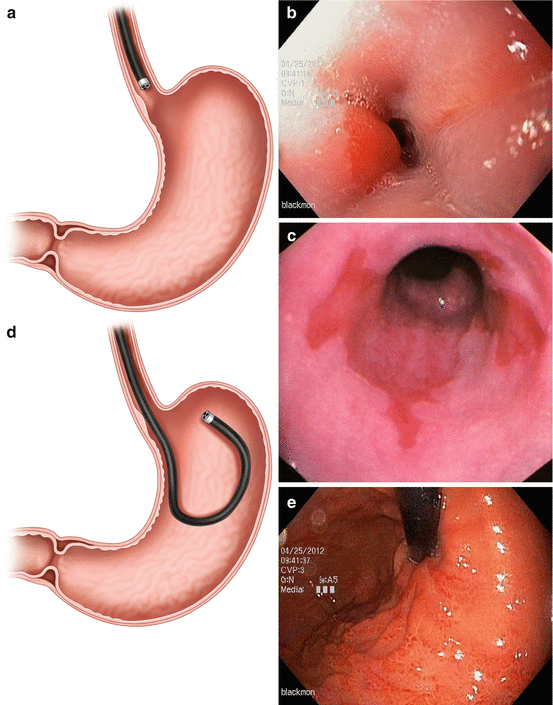
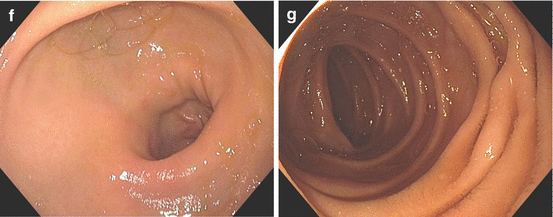
Fig. 3.3
The gastroesophageal junction is easily identified where the squamous esophagus lining transitions over to the columnar gastric mucosa (a, b). When this Z-line undergoes metaplasia, biopsies must be performed to confirm Barrett’s esophagus and rule out dysplasia (c). The gastroesophageal junction is visualized on retroview of the endoscope (d, e). The pyloric sphincter (f) and its intubation into the duodenum (g) must also be visualized and documented on every routine endoscopy
The esophagus frequently veers slightly to the left as it traverses the diaphragm. The gastroscope should be advanced under direct vision into the stomach; it should never be advanced when a lumen cannot be identified.
As the gastroscope is advanced into the stomach, the endoscopist must become oriented to the gastric anatomy. The lesser curvature of the stomach occupies the 12 o’clock position and the field of observation. The mucosa in this area is usually smooth. Careful observation in the lesser curvature is warranted, as this is frequently the site of gastric ulceration or malignancy.
The greater curvature occupies the 6 o’clock position. This area is characterized by prominent parallel rugae folds, which do not totally flatten with insufflation of air. The 9 o’clock position represents the anterior wall of the stomach, and the posterior wall is at the 3 o’clock position.
As the instrument is advanced further into the body of the stomach, the gastric incisura, or angularis, becomes visible on the lesser curvature. The smooth, arching fold separates the body from the antrum of the stomach. Alteration of the smooth contour of this fold may indicate previous ulcer disease or an infiltrative process.
Beyond the angularis lies the antrum of the stomach. The scope should be advanced into the antrum after careful observation of all the features of the gastric corpus. Typically, there are no rugal folds in this region. The pylorus lies at the distal extent of the antrum. It is worth waiting a moment after entering the antrum to watch the peristaltic waves pass the pylorus. The peristaltic activity is useful in unfolding all areas of the antrum for observation; and watching it also ensures that there is no infiltrative process limiting motility.
Once positioned at the angle of the stomach, the tip of the endoscope may be turned to view the gastric cardia and fundus (Fig. 3.3d, e). This retroflexed view is useful in identifying and treating lesions of the proximal stomach and distal esophagus. It is useful to twist the insertion tube of the instrument into this position to bring the area surrounding the scope into view. Subtle lesions of the fundus and cardia are best identified in this manner, so this maneuver must be part of every endoscopic examination of the stomach.
When examining a patient with esophageal cancer, one must ensure that the tumor does not extend into the gastric lumen. If cardia involvement is detected, the patient may not be able to have a gastric conduit and may instead require an alternate conduit such as colon or small bowel [1, 2].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








